AWES TGT Chemistry Mock Test - 5 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES TGT Chemistry Mock Test - 5
Recently, who won the National Women’s Carrom title for the twelfth time?
Who became the first-ever Indian to win a men's singles trophy at a World Table Tennis (WTT) Feeder Series event in Beirut, Lebanon?
Which company's rocket was used to launch the TSAT-1A satellite?
Initially, what was meant by mainstreaming children with special needs?
According to NEP 2020, what is the role of school counselors?
Schools should cater to individual differences to:
The reaction of SOCI2 on alkanols to form alkyl chlorides gives good yields because
What happens to atomic radius on going from left to right in a period in a periodic table?
Calculate the cell EMF in mV for
Pt|H2(1atm) |HCl(0.01M)|AgCl(s)| Ag(s) at 298 K
If ΔG°r values are at 25°C
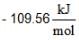 for AgCl(s) and
for AgCl(s) and 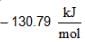 for H+ + Cl-) (aq)
for H+ + Cl-) (aq)
Johann Dobereiner classified elements in group of three elements called as:
Why some of the physical properties of solids show different values when measured along different directions in the same crystals?
For the equilibrium,
at 1000 K. If at equilibrium pCO = 10 then total pressure at equilibrium is
1 M NaCl and 1 M HCl are present in an aqueous solution. The solution is
I− reduces IO3- and I2 and itself oxidised to I2 in acidic medium. Thus, final reaction is
The number of radial nodes in 3s and 2p respectively are
How many different stereoisomers exist for the compound below ?
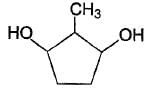
Statement I : cis-2-butene with cold, dilute, alkaline KMnO4 gives meso-2,3- butanediol.
Statement II : In alkaline solution, under cold condition, KMnO4 acts as a mild oxidising agent.
From the following, pick out the explanation for why molecule Y hydrolyses faster than molecule Z.
Direction (Q. Nos. 16-18) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Set of species with identical bond order is/are
Based on the following thermodynamic data,
Q. On the total mass basis of reactants, which reaction will generate the greatest amount of heat?
The increasing order of enthalpy of vaporization of NH3, PH3, and AsH3 is
Which one of the following is a water soluble vitamin?
Which is the best description of Hammond postulate?
The hydroxide of alkaline earth metal, which has the lowest value of solubility product (Ksp) at normal temperature (25°C) is-
The chemical reaction in which reactants require high amount of activation energy are generally
Catalytic converters must be used in cars to reduce the to reduce the effect of exhaust fumes on the atmosphere. The converter should contain one of the main components



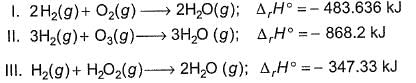 From I, total mass of reactant, 36 gm
From I, total mass of reactant, 36 gm










