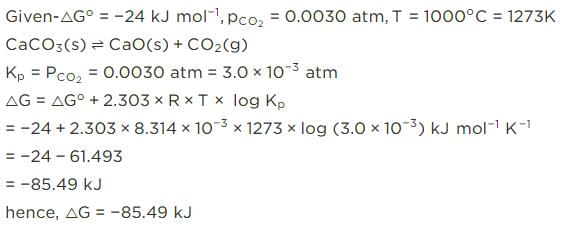AWES TGT Chemistry Mock Test - 8 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES TGT Chemistry Mock Test - 8
What is ‘Homosep Atom’, recently seen in the news?
Which of the following countries participated in ‘Exercise Dosti’, recently seen in the news?
Which country became the first to launch an electromagnetic railgun from an offshore vessel?
Recently, which organization has launched the ‘Global Initiative on Digital Health (GIDH)’?
Lough Neagh Lake, recently seen in the news, is located in which country?
Recently, NASA and which country’s space agency have joined to launch the world’s first wooden satellite?
Which organization has initiated legal action against OpenAI and Microsoft, alleging the unauthorized use of copyrighted content for training AI models, including ChatGPT?
In which country did the fertility rate hit a new low of 0.72 in 2023?
When a child with a disability first comes to school the teacher should?
Lead emitted by vehicles interferes with development of:
Oxidation number of 1/2 is assigned to oxygen atom in
One or More than One Options Correct Type
This section contains 5 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Select the correct point(s) of distinction between a volatic cell and electrolysis cell.
A gas absorbs a photon of 355 nm and emits at two wavelengths. If one of the emissions is at 680 nm, the other is at:
25.3 g of sodium carbonate, Na2CO3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, molar concentration of sodium ions, Na+ and carbonate ions, 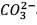 are respectively. (At. mass of carbon = 40)
are respectively. (At. mass of carbon = 40)
A metal M readily forms water soluble sulphate, and water insoluble hydroxide M(OH)2. Its oxide MO is amphoteric, hard and having high melting point. The alkaline earth metal M must be -
An electrochemical cell was based on the following reaction:
Mn(OH)2(s) + H2O2(aq) → MnO2(s) + 2H2O (e)
During the opeartion of this for 1 min, 0.135 g of MnO2 was produced. What is the average electric current (in ampere ) produced by the cell?
What are the products in the following reaction?
For the reaction, at 1000° C
ΔG° = - 24 kJ mol-1, 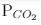 = 0.0030 atm.
= 0.0030 atm.
Q. Hence, ΔG at this temperature is
Number of neighbours and next - nearest neighbours of K respectively, are
Commercial concentrated nitric acid is 15.6 M. To prepare 10 L of 6.0 M nitric acid from it,
An aqueous solution contains 0.01 M RNH2 (Kb= 2 × 10-6) & 10-4 M NaOH.
The concentration of OH- is nearly:
The precipitate of CaF2 (Ksp = 1.7 × 10-10) is obtained when equal volumes of the following are mixed
Change in volume of the system does not alter the number of moles in which of the following equilibrium
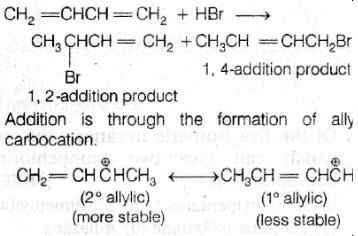
Reaction of one molecule of HBr with one molecule of 1,3–butadiene at 40ºC given predominantly
[AIEEE-2005]
Which of the following alkyl halides can produce only a single alkene product when treated with sodium methoxide?
Which quantum numbers gives the shell to which the electron belongs?




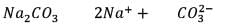
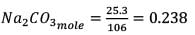
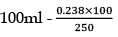
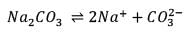
 0.955
0.955