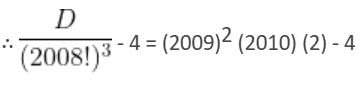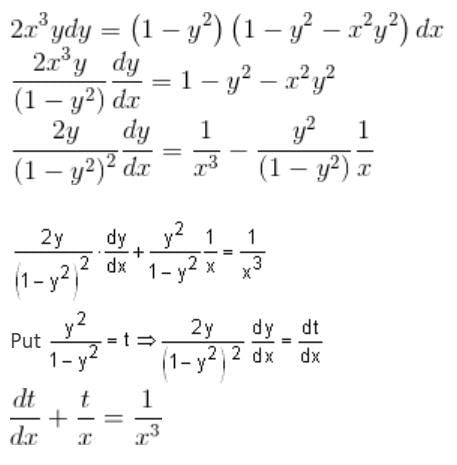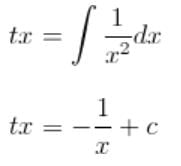BITSAT Mock Test - 10 - JEE MCQ
30 Questions MCQ Test BITSAT Mock Tests Series & Past Year Papers 2026 - BITSAT Mock Test - 10
Two masses M and m (with M > m) are connected by means of a pulley as shown in the figure. The system is released. At the instant when mass M has fallen through a distance h, the velocity of mass m will be


A diatomic molecule is made of two masses m1 and m2, which are separated by a distance r. If we calculate its rotational energy by applying Bohr's rule of angular momentum quantisation, then its energy will be given by
The following figure shows a spherical Gaussian surface and a charge distribution (magnitude of all the given point charges is different). When calculating the flux of electric field through the Gaussian surface, the electric field will be due to


Two identical thin rings, each of radius R, are coaxially placed at a distance R apart. If Q1 and Q2 are the charges uniformly spread on the two rings, the work done in moving a charge q from the centre of one ring to the centre of the other is
The de Broglie wavelength of a particle moving with a velocity 2.25 

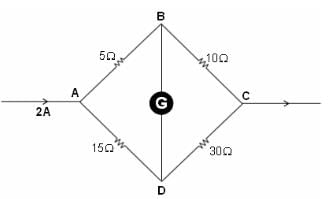
For the wheatstone bridge shown in the figure, total current is 2 A. What will be the current through BD?
A man standing midway between two cliffs, claps his hands and starts hearing a series of echoes at intervals of one second. If the speed of sound in air is 340 ms-1, the distance between the cliffs is
The formula mass of Mohr's salt is 392. The iron present in it is oxidised by KMnO4 in acidic medium. The equivalent mass of Mohr's salt is
Which of the following amines will not undergo carbylamine reaction?
If 10-4 dm3 of water is introduced into a 1.0 dm3 flask at 300 K, then how many moles of water are in the vapour phase when the equilibrium is established?
(Given: Vapour pressure of H2O at 300 K is 3170 Pa and R = 8.314 J K-1 mol-1)
At 25°C, the pH of a solution containing 0.10 M sodium acetate and 0.03 M acetic acid is
[pKa value of CH3COOH = 4.57]
Which of the following compounds, when heated with CO at 150oC and 500 atm pressure in the presence of BF3, forms ethyl propionate?
If the hydride ion (H-) is a stronger base than the hydroxide ion (OH-), then which of the following reactions will occur when sodium hydride (NaH) is dissolved in water?
Consider the following species:
(a) Acetate ion
(b) Salicylate ion
(c) Propanoic acid
(d) o-chloro phenol
The intramolecular hydrogen bonding is present in
Which of the following would be expected to have the largest entropy per mole?
Acidic buffer solution is produced on mixing the aqueous solutions of
Directions: A sentence has been given in Active/Passive Voice. Out of the four alternatives suggested, select the one which best expresses the same sentence in Passive/Active Voice.
Somebody should have cleaned the windows yesterday.
Directions: In the following question, a number series is given with one term missing. Choose the correct alternative that will continue the same pattern as established by the previous terms in the given series.
3, 5, 6, 10, 9, 15, 12, ___
Directions: The following question consists of a pair of words, which have a certain relationship with each other. Select the alternative which bears the same relationship as the original pair of words does.
FURY : IRE
Who among the following is taller than R but shorter than P?
The distance between two parallel planes 2x + y + 2z = 8 and 4x + 2y + 4z + 5 = 0 is
Nitika chose 12 numbers randomly. The sum of 12 numbers is 18 and the sum of their square is 30. What is the variance of the series?
If 
 is equal to
is equal to
If the sum of the squares of the intercepts on the axes cut off by the tangent to the curve x1/3 + y1/3 = a1/3 (a > 0) at 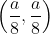 is 2, then the value of 'a' is
is 2, then the value of 'a' is
What is the solution of the differential equation 2x3y dy + (1 - y2)(x2y2 + y2 - 1) dx = 0?
|
2 videos|19 docs|70 tests
|
|
2 videos|19 docs|70 tests
|


 (M + m) v2
(M + m) v2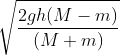
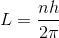
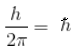



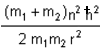

 . Here, the electric field E is due to charge inside the Gaussian surface only. Hence, the correct option is (4).
. Here, the electric field E is due to charge inside the Gaussian surface only. Hence, the correct option is (4).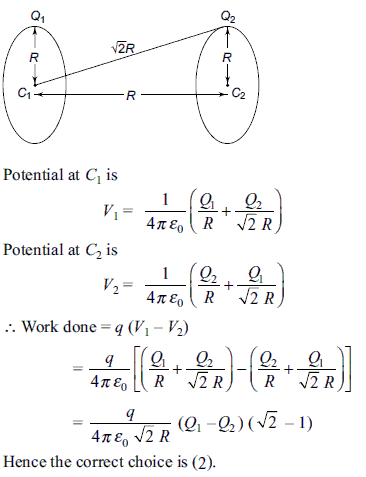

 5Fe3+ + Mn2+ + 4H2O
5Fe3+ + Mn2+ + 4H2O

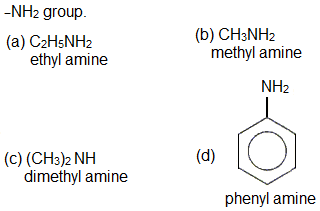
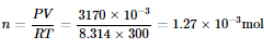

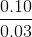
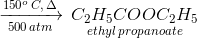

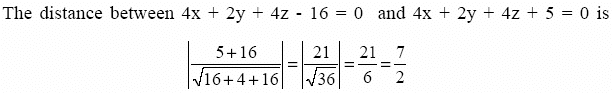
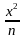 – (∑
– (∑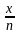 )2
)2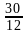 – (
– (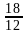 )2
)2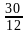 -
-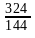
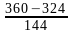
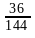 = 0.25
= 0.25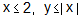 and
and  is
is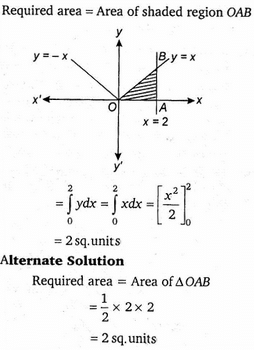
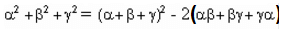
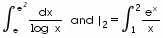 dx, then
dx, then
 I1 =
I1 =  =
= 
 I1 = I2
I1 = I2 , the constant term is
, the constant term is .
. is independent of x.
is independent of x. 45 - 5r = 0
45 - 5r = 0  r = 9
r = 9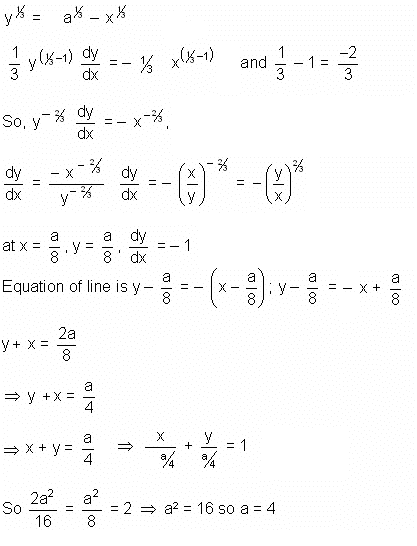
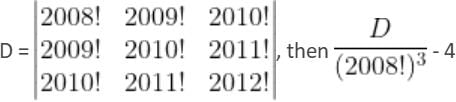 is divisible by
is divisible by
 R3 - R2; R2
R3 - R2; R2  R2 - R1
R2 - R1