BPSC AE Civil Paper 3 (General Studies) Mock Test - 1 - Civil Engineering (CE) MCQ
30 Questions MCQ Test - BPSC AE Civil Paper 3 (General Studies) Mock Test - 1
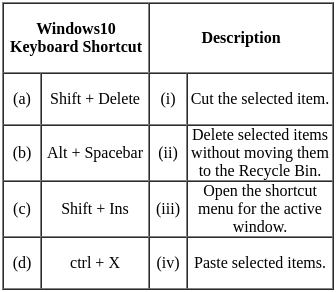
Match the column named 'Windows10 Keyboard Shortcut' with the column named 'Description'.

Which of the given options best describes the truth of the following statements?
i) An assembler is a program for converting instructions written in a high-level language into relocatable machine code, and generating information for the loader.
ii) The assembler must remain in main memory during the exaction of the assembly code.
iii) Different types of assemblers include One pass assemblers and load-and-go assemblers.
Which of the given options best describes the truth of the following statements?
i) Microsoft Outlook is an email application software.
ii) Email clients use mail access protocols such as POP/IMAP protocols to retrieve/sync emails from the server.
iii) Microsoft Exchange Server is a mail server and calendaring server developed by SunMicrosystems.
Who among the following wrote Ain-I Akbari?
Who developed the computer language COBOL?
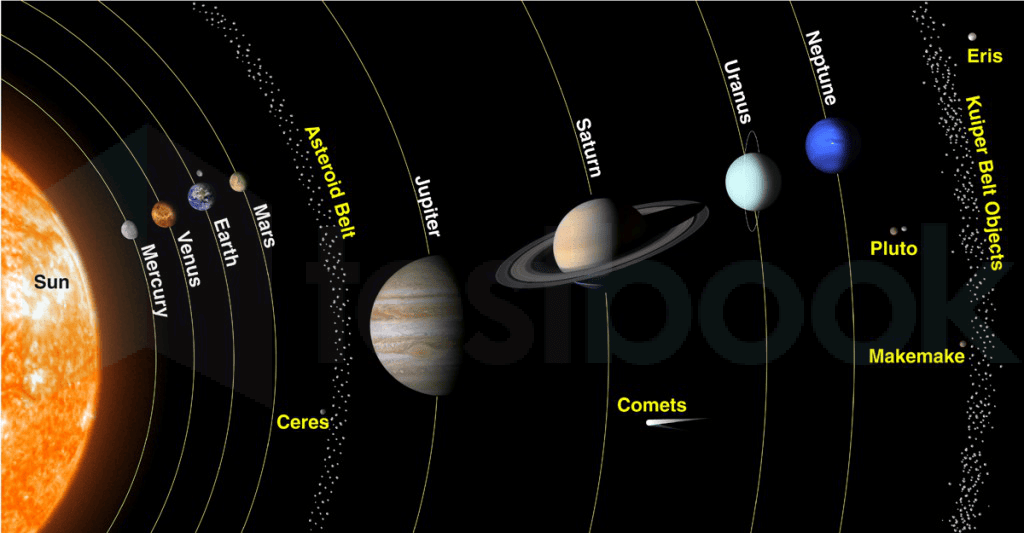
Which is the hottest planet of our solar system?
In general, a neutralization reaction can be written as:
Gold and silver are extracted from their respective ores by ________.
The "race to the bottom" phenomenon, often associated with globalization, implies:
What is the general stereoscopic division between the points in the free state?
Who discovered the vaccine for smallpox in 1798?
Who won the Batch Open Squash title by defeating France's Melvil Scianimanico?
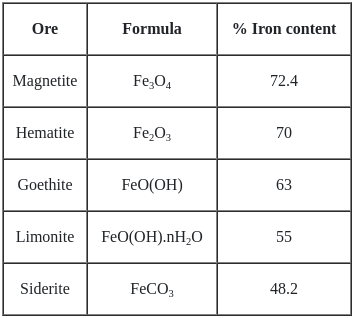
"Siderite" is the ore of which among the following metals ?
What is the quantitative study of products made in the required reactions or chemical reactions?
What is the full from of ASCII?
When a light undergoes reflection, the velocity of the reflected light is always:
Why goldsmiths use the outermost zone of a flame for melting gold and silver, considering the temperature distribution within a flame.
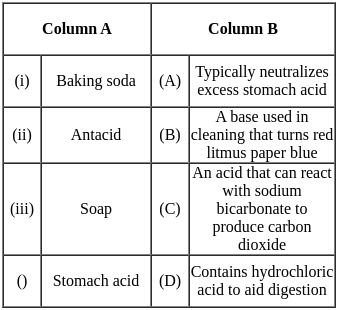
Match the substances with the correct descriptions of their reactions or properties in chemical terms, illustrating the varied interactions of acids and bases.
Consider a mixture of sand, iron filings, and salt. Describe a step-by-step method to separate each component.
The time difference between the actual sunset and apparent sun set is about:
Where will the maritime cooperative activity involving the defense forces of the US, Japan, Australia, and the Philippines take place?
Which of the following is/are incorrect about neutralisation reaction?
(A) It is a reaction between an acid and a base
(B) In this reaction, a new substance is formed, called salt
(C) Salts formed are only either acidic or basic in nature
(D) Heat is always absorbed in the reaction
Consider the following statements regarding euthanasia:
- Active euthanasia entails the use of substances to end the life of the patient.
- Passive euthanasia involves simply stopping lifesaving treatment or medical intervention.
- Both passive and active euthanasia are allowed in India.
Which of the statements given above are correct?
Consider the following statements regarding the right to privacy in India:
- The right to privacy is protected under Article 14 of the Indian Constitution.
- The Supreme Court described the right to privacy under Part III of the Constitution in K.S. Puttaswamy v. Union of India.
Which of the statements given above is/are correct?
Lubrication of all moving parts is essential to
Consider the following statements regarding the Gallantry Awards in India:
- Kirti Chakra: It is the second-highest peacetime gallantry award.
- Shaurya Chakra: It is awarded for gallantry, courageous action, or self-sacrifice while engaged in direct action with the enemy.
Which of the statements given above is/are correct?
Which one of the following is also called Stranger Gas ?
A _________ hides your IP address by redirecting your IP address across the network through a specially configured remote server run by the VPN host.
The study of human populations including their size, composition and distribution across space and the process through which populations change is called _______.
Four bulbs are having the rating 220 V 40 W, 220 V 60 W, 220 V 100 W and 220 V 18 W respectively. Which of the following bulbs has the least resistance?