Bihar PGT Chemistry Mock Test - 8 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test Bihar PGT Exam Mock Test Series 2025 - Bihar PGT Chemistry Mock Test - 8
In the following question, four words are given out of which one word is incorrectly spelt. Find the incorrectly spelt word.
‘लाल किले पर तिरंगा फहराया गया।’ इसके रेखांकित शब्द में कौन-सा समास होगा?
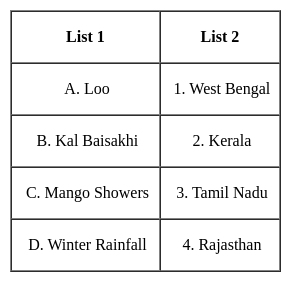
Match List 1 and List 2 and select the correct answer.
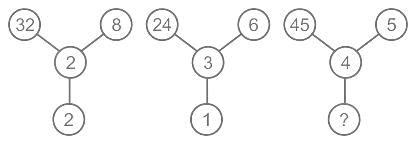
Select the number which can be placed at the sign of the question mark (?) from the given alternatives.
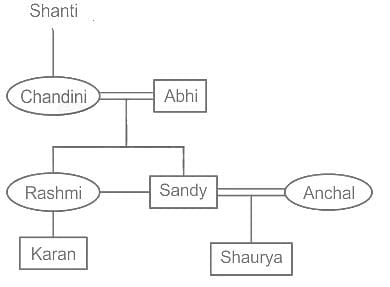
Shanti’s daughter Chandini is married to Abhi. Anchal is married to Sandy, the grandson of Shanti. Abhi's grandson is Karan. Rashmi is the mother of Karan. Shaurya is Anchal's son. How is Shaurya related to Karan?
Which of the following metal solution cannot be prepared by Bredig’s arc method?
Consider the following compounds
I. K4[Fe(CN)6]
II. NH4Cl
III. H2SO4
IV. [Ni (CO)4]
Q.
Ionic, covalent and coordinate bonds are present in
One or More than One Options Correct Type
This section contains 5 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Select the correct point(s) of distinction between a volatic cell and electrolysis cell.
In the following alcohol, which — OH group is involved to the maximum extent in H-bonding?
The element which shows only negative oxidation state/s among following elements is:
A definite amount of solid NH4HS is placed in a flask already containing ammonia gas at a certain temperature and 0.50 atm pressure. NH4HS decomposes to give NH3 and H2S and at equilibrium total pressure in flask is 0.84 atm. The equilibrium constant for the reaction is :
What is the major product in the following reaction?
An electrochemical cell was based on the following reaction:
Mn(OH)2(s) + H2O2(aq) → MnO2(s) + 2H2O (e)
During the opeartion of this for 1 min, 0.135 g of MnO2 was produced. What is the average electric current (in ampere ) produced by the cell?
According to MO theory which of the following lists ranks the nitrogen species in terms of increasing bond order?
A gas absorbs a photon of 355 nm and emits at two wavelengths. If one of the emissions is at 680 nm, the other is at:
A group of 14 element is converted into n – type semiconductor by dopping it with
Which one of the following exists in the oxidation state other than +3?
The specific rotation of optically pure (R)-2- butanol is -13.52° at 25°C. An optically pure sample of (R)-2- bromobutane was treated with aqueous NaOH in order to form 2-butanol via SN2 reaction. What would be the specific rotation of the product assuming 100% yield?
Which among the following statement is not true for rate constant of a reaction?
For which of the following parameters the structural isomers C2H5OH and CH3OCH3 would be expected to have the same values?
[AIEEE 2004]