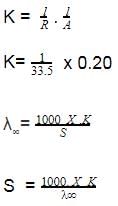Bihar STET Paper 2 Chemistry Mock Test - 4 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test Bihar STET Mock Test Series 2025 - Bihar STET Paper 2 Chemistry Mock Test - 4
A gas absorbs 200 J of heat and expands by 500 cm3 against a constant pressure of 2 x 105 Nm-2. Change in internal energy is
Which is the most suitable reagent for the following transformation?
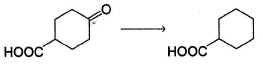
The nature of bonds in the compounds of carbon is mostly:
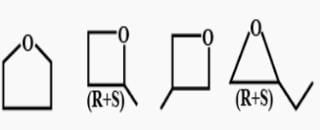
How many structural isomers exist for C4 H80 which are simultaneously ether? Also there is no atom sp2-hybridised.
What is aggregation of colloidal particles into insoluble precipitate by addition of some suitable electrolyte called?
Anti – histamines stop allergic reactions by
In the equation Kt = log C0 – log Ct, the curve between t and log Ct is -
[AIEEE-2002]
The half life of a reaction is halved as the initial concentration of the reactant is doubled. The order of the reaction is
Which is the product of the given reaction?
The solubility of [Co(NH3)4Cl2] CIO4_________ if the = 50,
= 70, and the measured resistance was 33.5Ω in a cell with cell constant of 0.20 is ____.
Find the number of waves made by a Bohr’s electron in one complete revolution in its 3rd orbit
An aqueous solution is 34% H3P04 by mass and has density 1.209 g mL -1 Molarity (I), molality (II) and normality (III) respectively, are
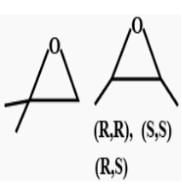
Which of the follownig compounds is (S)-4-chloro-1-methylcyclohexene ?
A pressure cooker reduces cooking time for food because _
[AIEEE-2003]
The standard reduction potential of a silver chloride electrode is 0.2 V and that of a silver electrode is 0.79 V. The maximum amount of AgCl that can dissolve in 106 L of a 0.1 M AgNO3 solution is
In which of the following complexes the nickel metal is in highest oxidation state.
What is the major monobromination product in the following reaction?
In allene (C3H4 ), the type(s) of hybridisation of the carbon atoms is (are)
[JEE Main 2014 Online Exam]
Which of the following amine will form stable diazonium salt at 273-283 K ?
Which of the following is a negative ligand?
An aqueous solution of a solute AB has boiling point of 101.08° C and freezes at -1 .80 °C . AB is found to be 100% ionised at boiling point. If Kb /Kf = 0.3, then AB
Identify the teacher-related factor that affects learning.
Shalini investigates a topic thoroughly and does not need to be over-directed. In which learning phase is she?
With whom has the Government of India signed a loan agreement to strengthen the Fintech eco system in India?
A journey of 192 km takes 2 hours less by a fast train than by a slow train. If the average speed of the slow train be 16 kmph less than that of fast train, what is the average speed of the faster train?