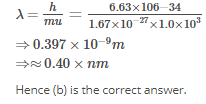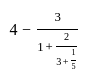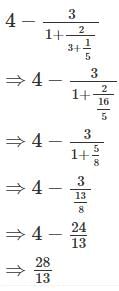Bihar STET Paper 2 Chemistry Mock Test - 6 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test - Bihar STET Paper 2 Chemistry Mock Test - 6
The correct order of thermal stability of the hydrides of group 16 elements is
Calculate the wavelength (in nanometer) associated with a proton moving at 1.0×103ms-1
(Mass of proton = 1.67×10-27 kg and h = 6.63×10-34 Js ) : [AIEEE 2009]
A hydrogen electrodes is immersed in a solution with pH = 0 (HCl). By how much will the potential (reduction) change if an equivalent amount of NaOH is added to the solution. (Take PH2 = 1 atm) T = 298 K.
Which of the following forms a colloidal solution in water?
For the reaction,
2SO2 (g) + O2 (g) 2SO3 (g) + 188.3 KJ
2SO3 (g) + 188.3 KJ
the number of moles of SO3 formed is increased if
Chlorine, bromine and iodine when combined with oxygen, have oxidation numbers
Sodium pentacyanonitrosylferrate(II) is also called?
When K2O is added to water, the solution becomes basic in nature because it contains a significant concentration of -
Among the following sets, highest boiling points are of the species.
I. HF, HCI, HBr, HI
II. H2O, H2S, H2Se, H2Te
III. NH3,PH3, AsH3,SbH3
The constant k used in rate equation is known as
Hydrogen has tendency to gain one electron to acquire helium configuration, in this respect it resembles:
Presence of particulate matter in polluted air catalyses the oxidation of sulphur dioxide to:
Which of the following elements are called representative elements?
The number of atoms in 0.1 mol of a triatomic gas is
For an aqueous solution, freezing point is _0.186ºC . Boiling point of the same solution is
(Kƒ = 1.86º K mol-1 kg) and Kb = 0.512º K mol-1 kg)
[AIEEE-2002]
In a class individual learners differ from each other in terms of:
Which of the following is not an activity aids related to teaching-learning materials?
Teaching learning materials, which are called teaching aids, have an advantage in which of the following forms?
To make assessment 'a useful and interesting process', one should be careful about:
In the villages of Bihar, many farmers do bee-keeping and collect honey to earn extra money. The best time to start bee-keeping is:
Sarita's teacher engages her in a number of group activities such as group discussions, group projects, etc. Which learning dimension is her teacher following?
If ‘r’% of ‘r’ is 49, what is the value of ‘r’?