Bihar STET Paper 2 Chemistry Mock Test - 8 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test Bihar STET Mock Test Series 2025 - Bihar STET Paper 2 Chemistry Mock Test - 8
Which among the following statement is not true for rate constant of a reaction?
A proton accelerated from rest through a potential difference of 'V' volts has a wavelength λ associated with it. An alpha particle in order to have the same wavelength must be accelerated from rest through a potential difference of
25.3 g of sodium carbonate, Na2CO3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, molar concentration of sodium ions, Na+ and carbonate ions, 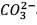 are respectively. (At. mass of carbon = 40)
are respectively. (At. mass of carbon = 40)
Consider the reactions
(A) H2O2 + 2HI → I2 +2H2O
(B) HOCl + H2O2 → H3O + Cl - - + O2
Which of the following statements is correct about H2O2 with reference to these reactions? Hydrogen perioxide is ________.
In an atom, an electron is moving with a speed of 600 m/s with an accuracy of 0.005%. Certainity with which the position of the electron can be located is (h = 6.6×10-34 kg m2s-1 ,mass of electron, em = 9.1×10-31 kg ) [AIEEE 2009]
Recycling of materials and energy will lead to
A definite amount of solid NH4HS is placed in a flask already containing ammonia gas at a certain temperature and 0.50 atm pressure. NH4HS decomposes to give NH3 and H2S and at equilibrium total pressure in flask is 0.84 atm. The equilibrium constant for the reaction is :
The curve showing the variation of adsorption with pressure at constant temperature is known as
In Dobereiner's Triads, elements were grouped based on their similar chemical properties. Which of the following elements was not part of any known Dobereiner's Triad?
Across the lanthanide series, the basicity of the lanthanide hydroxides
Kc forthe decomposition of NH4HS(s) is 1.8x 10-4 at 25°C.
If the system already contains [NH3] = 0.020 M, then when equilibrium is reached, molar concentration are
What are the roots of 1, 1, and 9 respectively as per the IUPAC nomenclature, and find out its symbol?
In a reaction, A + B → Product, rate is doubled when the concentration of B is doubled, and rate increases by a factor of 8 when the concentrations of both the reactants (Aand B) are doubled, rate law for the reaction can be written as
A coordination compound of cobalt has the molecular formula containing five ammonia molecules, one nitro group and two chlorine atoms for one cobalt atom. One mole of this compound produces three ions in an aqueous solution. The aqueous solution on treatment with an excess of AgNO3 gives two moles of AgCI as a precipitate. The formula of the complex and the isomerism shown by this is
In the following alcohol, which — OH group is involved to the maximum extent in H-bonding?
The equilibrium constant for the reaction
A(g) + 2B(g) 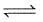 C(g) is 0.25 dm6 mol-2. In a volume of 5 dm3, what amount of A must be mixed with 4 mol of B to yield 1 mol of C at equilibrium
C(g) is 0.25 dm6 mol-2. In a volume of 5 dm3, what amount of A must be mixed with 4 mol of B to yield 1 mol of C at equilibrium
Which of the following carbonate of alkali metals has the least thermal stability ?
Azide ion exhibits an (N—N) bond order of 2 and may be represented by resonance structures I, II and III given below :
Select the correct statement(s) about more contributions,
Following equilibrium is set up at 298 K in a 1 L flask.
If one starts with 2 moles of A and 1 mole of B, it is found that moles of B and D are equal.Thus Kc is
The p-block elements constitute elements belonging to which groups?
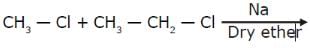
products.
Which of the following is not the free radical combination product in wurtz process.
Which of the following is/are the independent variable(s) of teaching?
The process of evaluation uses several methods because
The basic cause of a teacher`s failure in maintaining discipline is the lack of
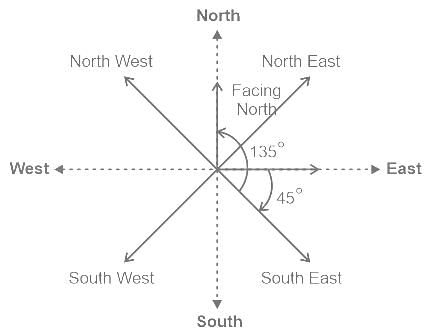
Sunny is facing East. After that, he turns 45° clockwise and then 135° anticlockwise. In which direction is he facing now?
From the given alternatives, select the word which CANNOT be formed using the letters of the given word.
FEARLESS



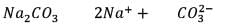
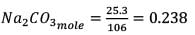
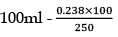
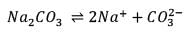
 0.955
0.955