Chemistry - 2015 Past Year Paper - IIT JAM MCQ
30 Questions MCQ Test - Chemistry - 2015 Past Year Paper
The first row transition metal complexes having tetrahedral geometry are high- spin due to
The species responsible for the superacidity of SbF5- HSO3F system is
A filter paper moistened with cadmium acetate solution turns yellow upon exposure to H2S. The transition responsible for the yellow colour is
The major product formed in the following reaction is
The structure of (2S,3R)- 2- amino- 3- hydroxy butanoic acid is
The ene- yne that produces a chiral compound upon treatment with Lindlar’s catalyst is
An organic compound P (C4H8O) is positive to Bayer¡¦s test, but inert to sodium metal. On treatment with conc. HCl, P gives CH3CH2Cl and CH3CHO. The structure of P is
Which one of the following is an identity matrix?
The intermolecular van der Waals potential is inversely proportional to r6. The corresponding force is proportional to
The normal spinel among the following mixed metal- oxides is
The ground state term for a free ion with 3d7 configuration is
The reagent ‘oxine’ commonly used in analytical chemistry is
The correct statement about ionization potential (IP) is
The set of products formed in the following reaction is
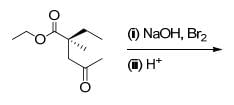
The correct set of reagents required for the following transformation is
The correct order of the pKa values for the conjugate acids of heterocyclic compounds given below is
The correct order of the 1H NMR chemical shift values for the indicated hydrogens (in bold) in the following compounds is
Which of the following statements are correct for SNAr reaction?
(i) Follows second order kinetics
(ii) KH/KD > 1
(iii) Involves carbanion- type intermediate
(iv) Involves two transition states
According to the equipartition principle, the predicted high temperature limiting value of the molar heat capacity at constant volume for C2H2 is
The major product formed in the following reaction is
At 25 °C, the solubility product (Ksp) of CaF2 in water is 3.2 × 10–11. The solubility (in mole per kg of water) of the salt at the same temperature (ignore ion pairing) is
For an isothermal free expansion of an ideal gas into vacuum, which one of the following set of values is correct?
The kinetics of the reaction 2N2O5 → 4NO2 + O2 in liquid bromine medium was measured independently for three different initial concentrations of N2O5: 0.11, 0.07 and 0.05 mol L–1. The half- life of the reaction was found to be 4.5 hours for all these concentrations. The order of the reaction is
The concentration of K+ ion inside a biological cell is 20 times higher than outside.The magnitude of potential difference between the two sides is [Given: 2.303 RT/F = 59 mV]
The correct order of the fundamental vibrational frequencies of the following diatomic molecules is
Identify the correct reagents required for the following transformation
The complex that is expected to show orbital contribution to the overall magnetic moment is














