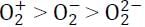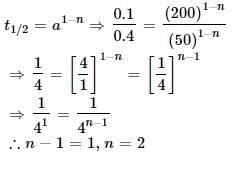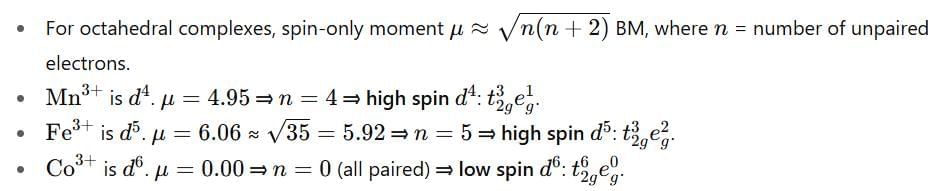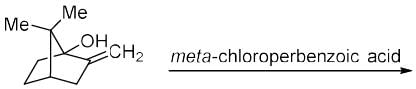Chemistry - 2019 Past Year Paper - IIT JAM MCQ
30 Questions MCQ Test - Chemistry - 2019 Past Year Paper
For a reaction of the type A + B → Products, the unit of the rate constant is mol L–1 s–1. The overall order of the reaction is ______.
The thermodynamic criterion for spontaneity of a process in a system under constant volume and temperature and in the absence of any work other than expansion work (if any) is:
The number of vibrational mode(s) of a carbon dioxide molecule that can be detected using infrared spectroscopy is ______.
For three non-coplanar vectors a, b and c, the expression a ∙ (b × c) can be written as:
The correct option for the metal ion present in the active site of myoglobin, hemocyanin, and vitamin B12, respectively, are:
The correct order of wavelength (λmax) of the halide to metal charge-transfer band of [Co(NH3)5Cl]2+ (I), [Co(NH3)5Br]2+ (II) and [Co(NH3)5I]2+ (III), is
The correct option for the major products of the following reaction is

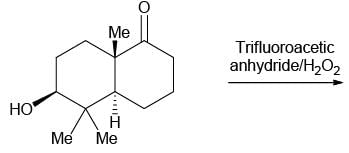
The major product formed in the following reaction is
The complementary strand for the following single strand of DNA is:
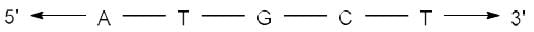
The correct option for the number of bending modes of vibration in each of H2O, CS2 and SO2 molecules, respectively, is
The total number of degrees of freedom of an HBr molecule that is constrained to translate along a straight line but does not have any constraints for its rotation and vibration is:
According to the kinetic theory of gases, the ratio of the root mean square velocity of molecular oxygen and molecular hydrogen at 300 K is:
The half-life of the chemical reaction, A → Product, for initial reactant concentrations of 0.1 and 0.4 mol L–1 are 200 and 50 s, respectively. The order of the reaction is:
The ratio of the nearest neighbor atomic distances in body-centered cubic (bcc) and facecentered cubic (fcc) crystals with the same unit cell edge length is
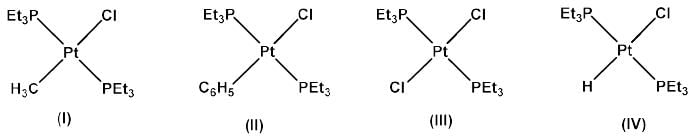
The correct trend in the rate of substitution of Cl- by pyridine in the following complexes is
In the qualitative inorganic analysis of metal ions, the ion which precipitates as sulfide in the presence of H2S in warm dilute HCl is:
The correct statement regarding the observed magnetic properties of NO, O2, B2, and C2 in their ground state is:
The observed magnetic moments of octahedral Mn3+, Fe3+ and Co3+ complexes are 4.95, 6.06 and 0.00 BM, respectively. The correct option for the electronic configuration of Mn3+, Fe3+ and Co3+ metal ions in these complexes, respectively, is
Among the following compounds, the one having the lowest boiling point is
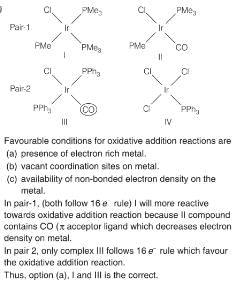
The correct option having one complex from each of the following pairs which is more reactive towards the oxidative addition reaction by hydrogen molecule is
Pair 1: IrCl(PMe3)3 (I) and IrCl(CO)(PMe3)2 (II)
Pair 2: IrCl(CO)(PPh3)2 (III) and IrCl3(PPh3) (IV)
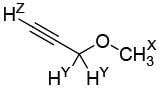
In 1H NMR spectrum of the given molecule, the correct order of chemical shifts of the labelled protons (HX, HY, HZ) is
In the following reaction of (D)-Glucose, a product P is formed.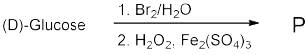
Among the following compounds, the one which will give the same product (P) under identical reaction conditions is
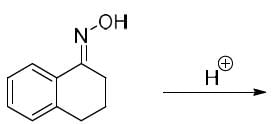
The correct option for the product(s) of the following reaction is
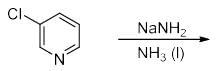
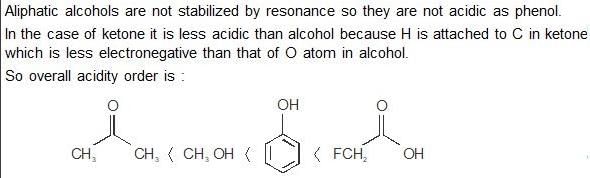
The increasing order of acidity of the given molecules in aqueous media is:
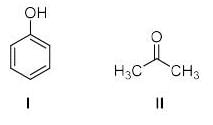
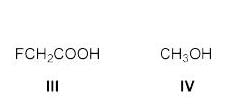
The compound formed upon subjecting an aliphatic amine to Lassaigne’s test is