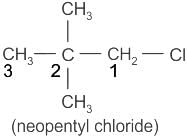
Chemistry: CUET Mock Test - 6 - CUET MCQ
30 Questions MCQ Test CUET UG Mock Test Series 2026 - Chemistry: CUET Mock Test - 6
Drugs that block the binding site of an enzyme for a substrate are called ______
Among the following statements, choose the correct statements.
A. SN2 reaction proceeds with stereo chemical inversion.
B. The process of conversion of Racemic mixture into enantiomer is known as Racemisation
C. A mixture containing 2 enantiomers in equal proportions is known as Racemic mixture.
D. The stereoisomers related to each other as superimposable mirror image are called enantiomers.
E. The objects which are non- superimposable on their mirror image are said to be chiral and this properly is known as chirality.
Choose the correct answer from the options given below:
Among the following statements, choose the correct statements.
A. Boiling point of alcohols increases with increase in the number of carbon atoms.
B. In alcohols, boiling points increases with increase of branching in carbon chain.
C. Boiling points of alcohols are lesser in comparison to haloalkanes of comparable molecular mass.
D. Boiling points of alcohols are higher in comparison to hydrocarbons of comparable molecular mass.
E. The high boiling points of alcohols are mainly due to the presence of intermolecular hydrogen bonding.
Choose the correct answer from the options given below:
Which reagent is used to form alcohol from aldehyde?
What property is shared by most transition metals as mentioned in the passage?
What is the main reason transition metals can exist in multiple oxidation states?
Which of the following is a typical property of transition metals?
What type of isomerism occurs when ligands are arranged differently in space, such as in cis and trans configurations?
What does stereoisomerism refer to in coordination compounds?
Which type of isomerism involves the arrangement of ligands in the same bonding configuration but different spatial orientations?
What is the characteristic of optical isomerism in coordination compounds?
Which of the following is an example of stereoisomerism in coordination compounds?
Consider the following statements regarding the factors affecting the rate of a reaction:
(A) Higher temperature increases the frequency of collisions between reactant molecules.
(B) Catalysts increase the rate of reaction by lowering the activation energy.
(C) The presence of a catalyst alters the equilibrium position of a reaction.
(D) Increased concentration of reactants generally increases the rate of reaction.
Choose the correct statements:
Consider the following statements regarding the rate law:
(A) The rate law expresses the rate of a reaction in terms of the concentration of reactants.
(B) The rate constant (k) depends on the concentration of reactants.
(C) The rate law is determined experimentally, not from the stoichiometry of the reaction.
(D) For a first-order reaction, the rate constant has units of time⁻¹.
Choose the correct statements:
A certain compound occupied a site Y of an enzyme near to the active site. This immediately resulted in the change of shape of the active site. Y is called a/an ______
Which of the following is incorrect regarding receptors?
Which of the following is not a reason for the selectivity of receptors towards messengers?
If the bond between the enzyme and inhibiting drug is very strong, which of the following takes place?
Which of the following is known as fight or flight hormone?
Which hormone plays an important role during child birth and post it?
Which hormone controls the balance of water and minerals in the body?
Which of the following best describes a particular enzyme?
|
39 docs|145 tests
|