Chemistry Mock Test- 1 - Class 10 MCQ
30 Questions MCQ Test - Chemistry Mock Test- 1
The blindness and death is caused by consuming adulterated liquor contains.
The property of metal by which it can be drawn into wires is called
Which of the following statements is correct about an aqueous solution of an acid and of a base ?
(i) Higher the pH, stronger the acid
(ii) Higher the pH, weaker the acid
(iii) Lower the pH, stronger the base
(iv) Lower the pH, weaker the base
(i) Higher the pH, stronger the acid
(ii) Higher the pH, weaker the acid
(iii) Lower the pH, stronger the base
(iv) Lower the pH, weaker the base
Mineral acids are stronger acids than carboxylic acids because
(i) mineral acids are completely ionised.
(ii) carboxylic acids are completely ionised.
(iii) mineral acids are partially ionised.
(iv) carboxylic acids are partially ionised.
Which of the following metals does not displace H2 gas from dilute HCl or dilute H2SO4?
Which one of the following metal reacts vigorously with oxygen and water?
Which of the following represents the saponification reaction ?
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that:
Which of the following metals will not react with oxygen,even when heated very strongly in air?
A virtual, erect and magnified image of an object is to be produced with a concave mirror of focal length 12 cm. Object may be placed at a distance of:
The graph of the polynomial p(x) intersects the x-axis three times in distinct points, then which of the following could be an expression for p(x):
Which of the following is not included in the assets of a commercial bank in India?
With Reference to the management of minor minerals in India, consider the following statements:
1. Sand is a ‘minor mineral’ according to the prevailing law in the country.
2. State Governments have the power to grant mining leases of minor minerals, but the powers regarding the formation of rules related to the grant of minor minerals lie with the Central Government.
3. State Governments have the power to frame rules to prevent illegal mining of minor minerals.
Q. Which of the statements given above is/are correct?
The property of metals by which they can be beaten in to thin sheets is called-
Consider the following statements:
1. Most of India’s external debt is owed by governmental entities.
2. All of India’s external debt is denominated in US dollars.
Q. Which of the statements given above is/are correct?
In the reaction, identify the reactant X.
CH3COOH + X CH3COOC2H5 + H2O
Ethanoic
acid
The oxidising agent used to convert alcohols into carboxylic acid is:
CH3CH2OH 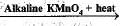 CH3COOH
CH3COOH
In the above given reaction, alkaline KMnO4 acts as
















