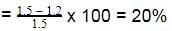Chemistry: Topic-wise Test- 2 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series - Updated 2026 Pattern - Chemistry: Topic-wise Test- 2
Compound with maximum ionic character is formed from :
Which of the following has a geometry different from the other three species (having the same geometry)?
Which of the following molecule does not have open book structure.
The hybridisation and geometry of BrF3 molecules are :
Which of the following species is not a pseudohalide ?
An inorganic white crystalline compound (A) has a rock salt structure (A) on reaction with conc. H2SO4 and MnO2, evolves a pungent smelling, greenish-yellow gas (B). Compound (A) gives white ppt. of (C) with AgNO3 solution. Compounds (A), (B) and (C) will be respectively
In the manufacture of sulphuric acid by contact process Tyndall box is used to
50.0 mL of 0.10 M HCl is mixed with 50.0 mL of 0.10 M NaOH. The solution temperature rises by 3.0°C Calculate the enthalpy of neutralization per mole of HCl. [take proper assumptions]
The enthalpy of neutralisation of a weak acid in 1 M solution with a strong base is -56.1 kJ mol-1. If the enthalpy of ionization of the acid is 1.5 kJ mol-1 and enthalpy of neutralization of the strong acid with a strong base is -57.3 kJ equiv-1, what is % ionization of the weak acid in molar solution (assume the acid to be monobasic) ?
For the allotropic change represented by the equation C (graphite) → C (diamond), ΔH = 1.9 kJ. If 6 g of diamond and 6 g of graphite are separately burnt to yield CO2, the heat liberated in first case is
The net heat change in a chemical reaction is same whether it is brought about in two or more different ways in one or several steps. It is known as -
According to Hess's Law the thermal effect of a reaction depends on -
How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -
How many dichlorocyclohexane would be produced upon free radical chlorination of chlorocyclohexane?
Arrange the following in increasing order of boiling points.
I. 3 -methyl pentane
II. 3-chloropentane
III. 3-bromopentane
IV. 3,3-dichloropentane
Direction (Q. Nos. 12 -15) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. In the following free radical bromination reaction, the important product(s) is/are
What is/are true regarding free radical iodination of an alkane?
Consider the following reaction.
The major product is
Terminal alkyne being therm odynamically less stable than an internal alkyne, instead when 2,2-dibromo butane is treated with NaNH2,1-butyne is formed as major product, not 2-butyne because
Direction (Q. Nos. 13 - 16) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : When 1-butyne is treated with NaNH2, sodium salt is formed.
Statement II : NH3 is w eaker acid than .
Statement l : C6H5MgBr with propyne gives 1-phenyl propyne.
|
1 videos|19 docs|71 tests
|