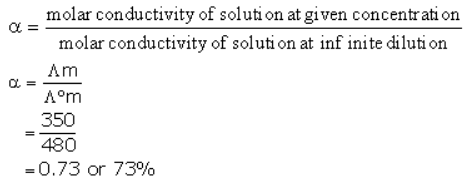Chemistry: Topic-wise Test- 4 - NEET MCQ
30 Questions MCQ Test - Chemistry: Topic-wise Test- 4
The number of moles of acidified KMnO4 required to convert one mole of sulphite ion into sulphate ion is-
N2(g) + 3H2 (g) 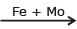 2NH3(g) ; Haber's process, Mo is used as -
2NH3(g) ; Haber's process, Mo is used as -
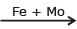 2NH3(g) ; Haber's process, Mo is used as -
2NH3(g) ; Haber's process, Mo is used as -Potash alum is a double salt, its aqueous solution shows the characteristics of-
The vapour pressure of n-hexane at 350 K is 840 torr and that of cyclohexane is 600 torr. Mole fraction of hexane in the mixture that boils at 350 K and 1 atm pressure assuming ideal behaviour is
12.8 g of naphthalene (C10H8) is dissolved in 92 g toluene (C7H8). Boiling point of this solution is
(boiling point of toluene = 110°Cand mole fraction boiling point elevation constant of toluene is 36.1 K)
Elevation in boiling point of a molar (1 M) glucose solution (d = 1.2 g mL-1) is
For a dilute solution containing 2.5 g of a non-volatile non-electrolyte solute in 100 g water, the elevation in boiling point at 1 atm pressure is 2°C. Assuming concentration of solute is much lower than the concentration of solvent, the vapour pressure (mm of Hg) of the solution is (Take, Kb = 0.76 K mol-1 kg).
[IITJEE2012]
Osmotic pressure of insulin solution at 298 K is found to be 0.0072 atm at 298 K. Hence, height of water column due to this pressure is (density of mercury = 13.6 g mL-1)
Which has maximum osmotic pressure at temperature T K?
There is formation of Prussian blue when Fe3+ reacts with [Fe(CN)6]4-. Two solutions as shown are separated by a semipermeable membrane AB. Due to osmosis, there is
Three solutions of following in water are isothermal conditions as given:
Thus,
Only One Option Correct Type
This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
The degree of dissociation (α ) o f weak electrolyte AxBy is related to van’t Hoff factor (i) by the expression
[AIEEE 2011]
0.004 M Na2S04 aqueous solution is isotonic with 0.01 M glucose solution at 300 K. Thus, degree of dissociation of Na2S04 is
When 20 g of naphthoic acid (C11H8O2) is dissolved in 50 g of benzene, a freezing point depression of 2 K is observed. [Kf (benzene) = 1.72Kmol-1 kg]. The van’t Hoff factor (i) is
[IIT - JEE 2007]
An aqueous solution of a solute AB has boiling point of 101.08° C and freezes at -1 .80 °C . AB is found to be 100% ionised at boiling point. If Kb /Kf = 0.3, then AB
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
The correct order of acidic strength of the following alcohols is
Which compound given below has the highest solubility in water ?
What is the correct increasing order of acidity of the following?
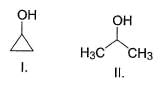
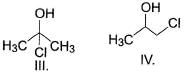
What is the order of solubility of the following in water?
Which of the following can be used for the distinction of ethanol from phenol?
Consider the following reaction,
The above reaction can best be brought about by
E° values of Mn+ + ne- → M are given below
Fe2+/Fe -0.44 V, Mg2+/Mg -2.37 V, Pt(H2)/H+ 0.00 V,
AI3+/AI -1.66 V , Zn2+/Zn - 0.76 V, Cu2+/Cu +0.34 V,
Ag+/Ag + 0.80 V, Ni2+/Ni -0.26 V
How many of the metals can be used to prevent corrosion of Ni to Ni2+?
W g of Ag is deposited at the cathode of one electrolytic cell due to passage of 1A of current for 1h.Time required for passage of current to deposit W g of Mg by the same value of current is
At 300K molar conductivity of solution A is 350 units, and at infinite dilution the molar conductivity of the same sample is 480 unit. Predict the percentage dissociation of the electrolyte.
With increase in temperature the degree of dissociation of electrolyte: