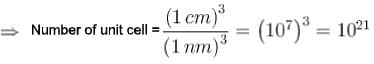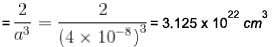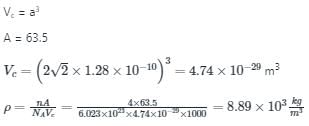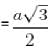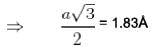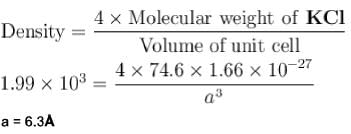Crystals NAT Level – 1 - Physics MCQ
10 Questions MCQ Test - Crystals NAT Level – 1
A crystal belongs to a face cubic lattice with four atoms in the unit cell. The size of crystal is 1cm and its unit cell dimension is 1nm.Then the number of atoms in the crystal is in unit of 1021 is?
Metallic monovalent sodium crystallizes in bcc structures. If the length of unit cell is 4 x 10-8 cm. The concentration of conduction electrons in metallic sodium is x * 1022 cm-3 . Find x?
Atomic radius of copper (fcc) is 1.28Â , then density of copper is (kg/m3 )
Nearest neighbour distance in Na crystal is 1 . 8 3  Density of electrons in Na crystal in m-3 is :
Density of electrons in Na crystal in m-3 is :
Molecular weight of KCI (fcc) is 74.6amu and its density is 1.99 x 103 kg/m3 . The lattice constant of KCI in units of 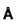 is :
is :
Smallest angle of diffraction for an X-ray beam of wavelength 0.71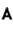 is (in degrees).
is (in degrees).
What is the approximate lattice constant “a” of a substance having FCC lattice, molecular weight 60.2 and density 6250 kg/m3 ? (Consider N = 6.02 x 1026 /kg-mole)
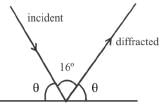
If the angle between direction of X-ray (incident) and diffracted one is 16°, the angle of incidence in degrees will be :
If 0.28nm is spacing between the nearest neighbouring ions in NaCI lattice, the unit cell parameter in 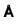 is?
is?
If the miller indices for a plane is [1 1 1] and 2 be the lattice constant, then what is the area of the plane ?