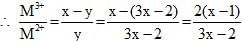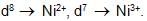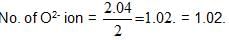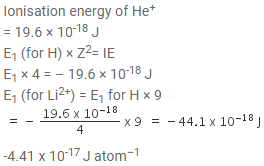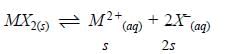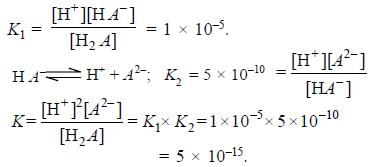DSSSB PGT Chemistry Mock Test - 9 - DSSSB TGT/PGT/PRT MCQ
30 Questions MCQ Test DSSSB PGT Mock Test Series 2025 - DSSSB PGT Chemistry Mock Test - 9
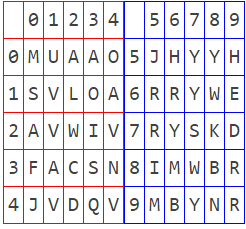
Two matrices are shown in the figure below. Their rows and columns are labelled as (0,1,2,3,4) and (5,6,7,8,9) in the manner shown. Find the correct row-column pairs out of the following matrices that decode to the word - VARY
Direction: Read the following passages carefully and answer the question that follows.
"Cloning is an advanced technological invention for producing a genetic twin of a living thing, an organism that starts life with the same genes as its parents. In mammals, DNA is taken from an adult animal and then it is inserted into an egg cell from another animal. This egg then divides into an embryo. The embryo is then transplanted into a surrogate mother and grown to term. This process has worked in animals like cows, sheep, goats, mice, pigs, rats, cat, monkeys, and horses." The opponents of human cloning say that an embryo at any stage of development is a human life. worthy of protection and any kind of research that entails destroying an embryo is immoral, unethical, no matter how worthy the intent may be. It involves using human beings as means; it turns human life into a commodity and fosters a culture of dehumanization.
Q. Find out the false statement out of the given options.
"Cloning is an advanced technological invention for producing a genetic twin of a living thing, an organism that starts life with the same genes as its parents. In mammals, DNA is taken from an adult animal and then it is inserted into an egg cell from another animal. This egg then divides into an embryo. The embryo is then transplanted into a surrogate mother and grown to term. This process has worked in animals like cows, sheep, goats, mice, pigs, rats, cat, monkeys, and horses." The opponents of human cloning say that an embryo at any stage of development is a human life. worthy of protection and any kind of research that entails destroying an embryo is immoral, unethical, no matter how worthy the intent may be. It involves using human beings as means; it turns human life into a commodity and fosters a culture of dehumanization.
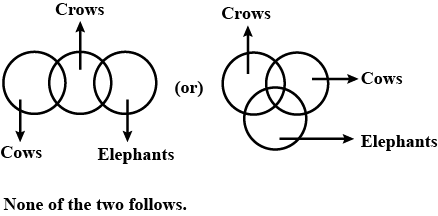
Statements:
Some cows are crows.
Some crows are elephants.
Conclusions:
1. Some cows are elephants.
2. All crows are elephants.
Some cows are crows.
Some crows are elephants.
1. Some cows are elephants.
2. All crows are elephants.
What is the no of zeroes at the end of the product of the no from1 to 100 ?
A transition Metal M can exist in two oxidation states +2 and +3.It forms an oxide whose experimental formula is given by MxO where x < 1. Then the ratio of metal ions in +3 state to those in +2 state in oxide is given by :
Analysis show that nickel oxide consist of nickel ion with 96% ions having d8 configuration and 4% having d7 configuration. Which amongst the following best represents the formula of the oxide.
Number of neighbours and next - nearest neighbours of K respectively, are
Which of the following sets of quantum numbers represents the highest energy of an atom ? [AIEEE 2007]
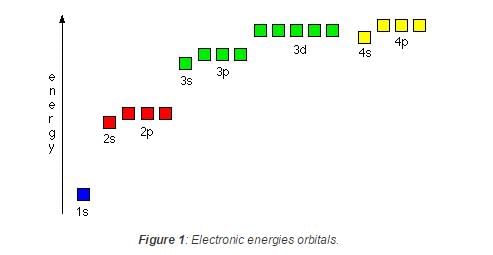
Ionisation energy of He+ is 19.6 x 10-18 J atom-1. The energy of the first stationary state (n = 1) of Li2+ is
[AIEEE 2010]
The solubility product of a salt having general formula MX2, in water is 4x10-12. The concentration of M2+ ions in the aqueous solution of the salt is - [AIEEE-2005]
The first and second dissociation constants of an acid H2A are 1.0 x 10-5 & 5.0 x 10-10 respectively. The overall dissociation constant of the acid will be -
[AIEEE-2007]
Direction (Q. Nos. 17) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Match the compounds/ions having underlined atoms of different oxidation number (in Column I) with values (in Column II).
The temperature coefficient of a standard Cd-cell is – 5.0X10– 5 Vk– 1 whose emf at 25°C is 1.018 V. During the cell operation, the temperature will -
A cell Ag | Ag+ || Cu++ | Cu initially contains 2M Ag+ and 2M Cu++ ions. The charger in cell potential after the passage of 10 amp current for 4825 sec is:
Aqueous solution of barium phosphate which is 100% ionised has ΔTf / Kf as 0.40. Hence, given solution is x * 10-2 molal. What is the value of x?
Direction (Q. Nos. 17-20) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Which of the following can be calculated based on Born-Haber cycle of formation of a lattice A+B- (s) from A(s) and 6 (g) ?
Which of the following Lewis acid-Lewis base interactions are associated with the expansion of octet?
PCIx F5 - x shows non-polar behaviour when x is
NO2 can be represented as
Q. Formal charge on each oxygen atom is
The complex which exhibits cis-trans isomerism as well as can be resolved into d and ℓ forms:
Which of the following structures represents a chiral compound ?
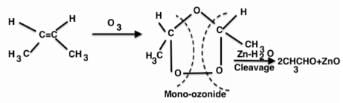
In the following sequence of reactions, the alkene affords the compound ‘B’
The compound B is
[AIEEE 2008]
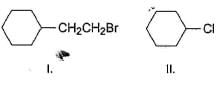
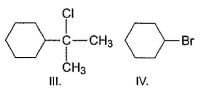
Arrange the following in increasing order of reactivity in an SN2 reaction with K I in acetone solvent.
Comprehension Type
Direction (Q. Nos. 12-14) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Consider the following reaction. The tertiary butyl group can be cis or trans to tosylate group. Answer the following questions based on the knowledge of E2 and E1 reaction mechanism.
Q.
The correct statement concerning above elimination reaction is
Comprehension Type
Direction (Q. Nos. 13-15) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Passage
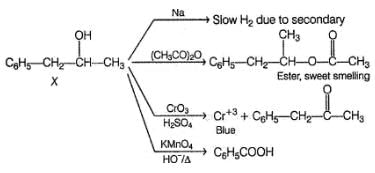
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
The structure of X is
Which of the following could result as a product in the aldol condensation reaction?
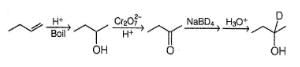
Consider the following reaction,
Q.
The most likely organic product X is
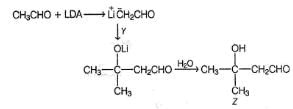
Consider the following reaction,
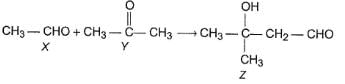
Q.
How the product Z can be prepared selectively using X and Y and other reagents?