EMRS PGT Chemistry Mock Test - 3 - EMRS MCQ
30 Questions MCQ Test - EMRS PGT Chemistry Mock Test - 3
In an examination Mohit got 30% of the maximum marks but failed by 25 marks. Another student who scored 38% got 15 marks more than the pass marks. The necessary pass percentage required is:
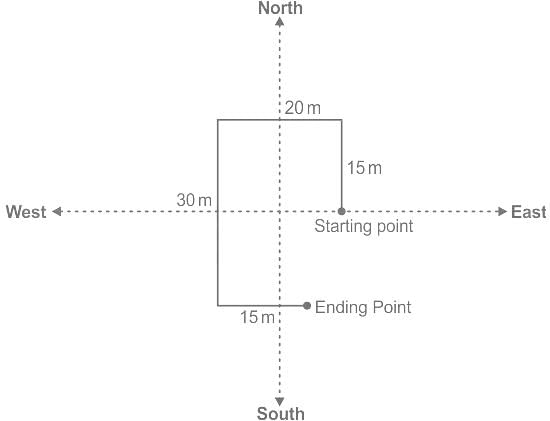
Ramu walks 15m towards the north then turns left and covers 20m then covers also 30m by turning south then again turns left and covers 15m. What is the total distance covered by him?
If a Paper (Transparent Sheet ) is folded in a manner and a design or pattern is drawn. When unfolded this paper appears as given below in the answer figure. Choose the correct answer figure given below.
A paper is folded as shown in the given figures and a cut is made. When opened how will it appear ? Choose from the given responses.
Question Figure
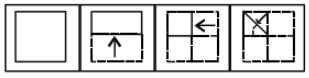
Answer Figure
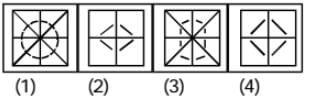
A paper is folded as shown in the given figures and a cut is made. When opened how will it appear ? Choose from the given responses.
Question Figure


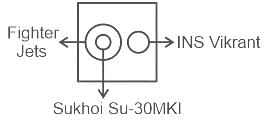
Identify the diagram that best represents the relationship among classes given below:
Fighter Jets, Sukhoi Su-30MKI, INS Vikrant
Information Communication Technology (ICT) doesn’t includes ______.
An cation A3+ has 18 electrons. Write the atomic number of A.
Consider the following reaction 2A + 3F2 → 2AF3.
What is the formula for the reaction product if we substitute iodine for fluorine?
Pick out the most reactive alkyl halide for an SN1 reaction.
Silver metal crystallises in a cubic closest packed arrangement with edge length 407 pm. Thus, radius of the silver atom is
Which of the ligand can show linkage isomerism and acts as flexidentate ligand:
At 87°C, the following equilibrium is established
H2(g) + S(s) 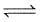 H2S(g) Kp = 7 × 10-2
H2S(g) Kp = 7 × 10-2
If 0.50 mole of hydrogen and 1.0 mole of sulfur are heated to 87°C in 1.0 L vessel, what will be the partial pressure of H2S at equilibrium ?
1 c.c. of 0.1N HCl is added to 99 CC solution of NaCl. The pH of the resulting solution will be
Among the following statements the one that is not true about Mendeleev’s Periodic Table is:
Gibbs free energy change for a cell reaction is positive what does it indicates?
An element has configuration 4d55s2. The element belongs to
The correct order of in creasing order of radiiions Br- , F-, O2- and S2- is as follow
Which one of the following is a form of Sternberg's triarchic theory of intelligence?
"A young child responds to a new situation on the basis of the response made by him/ her in a similar situation as in the past". This is related to:
In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.
Q. A situation that requires a lot of hard work.
In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.
Q. A person controlled by an evil spirit.