EMRS PGT Chemistry Mock Test - 4 - EMRS MCQ
30 Questions MCQ Test - EMRS PGT Chemistry Mock Test - 4
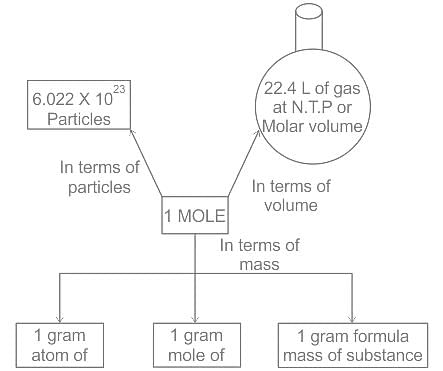
Among the following, the one with the highest mass is:
Which one of the following is not a function of roots for the plant ?
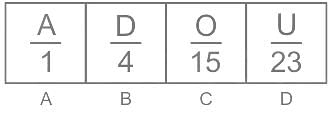
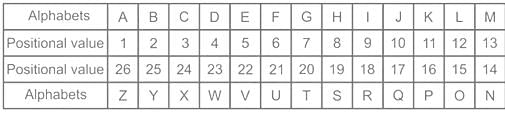
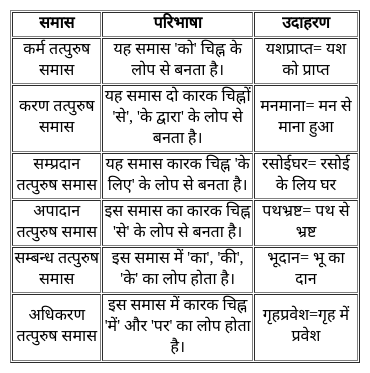
Select the number which can be placed at the sign of the question mark (?) from the given alternatives.
How many such 5's are there in the following numbers sequence, each of which is preceded by 3 or 4 but not immediately followed by 8 or 9?
35954553584567357554523510
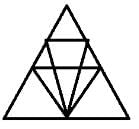
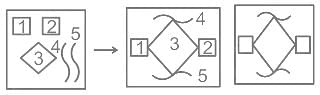
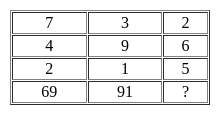
Which of the figures (1), (2), (3) and (4) can be formed from the pieces given in figure?
Problem Figure
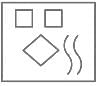
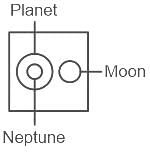
Select the Venn diagram that best illustrates the relationship among the following classes.
Neptune, Planet, Moon
Mrs. Kapoor wants to gift a digital album to her friend on her birthday. All the images are static and have lots of colour shading. Which of the following format is best suited for this purpose?
Which of the following complex will give white precipitate with barium chloride solution?
The second and third period of the modern periodic table contains how many elements in all?
If 40 ml of 0.2 M KOH is added to 160 ml of 0.1 M HCOOH [Ka = 2 × 10-4]. The pOH of the resulting solution is
Mean bond enthalpy of different bonds are given
Out of the given pairs, which compound is more stable than the other?
Consider the following elimination reaction,
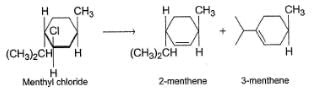
When reaction is carried out with C2H5ONain ethanol, 2-menthene is the major product while 3-menthene is the major product if reaction is substrate is heated in ethanol only. It is due to
An aqueous solution of a solute AB has boiling point of 101.08° C and freezes at -1 .80 °C . AB is found to be 100% ionised at boiling point. If Kb /Kf = 0.3, then AB
Consider the following oxidation/reduction process,
Q. Magnetic moment does not change in
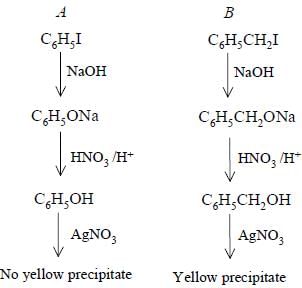
Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.
Which one of the following statements is true for this experiment ?
[AIEEE-2003]
Pick out the compound which reacts fastest in the presence of AgNO3.
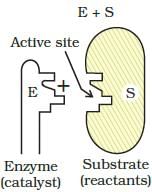
Which step of enzyme catalysis mechanism is shown in figure?
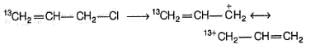
Statement I : When 3-bromo propene, which contain a labelled 13C at C-1 position is refluxed with methanol, following products were obtained.
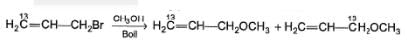
Statement II : Methanol has an acidic proton bonded to oxygen.
In the given questions, fill in the blanks with the appropriate words from the alternatives provided.
Even when the religious leader was arrested for a hideous crime, he still had the nerve to act __________ before the public.
In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.
Q. Something that cannot be easily viewed or felt.
In the following question, out of the four alternatives, select the alternative which best expresses the meaning of the idiom/Phrase.
Q. Bury the hatchet