EMRS PGT Chemistry Mock Test - 5 - EMRS MCQ
30 Questions MCQ Test - EMRS PGT Chemistry Mock Test - 5
Soil contains decayed remains of living organisms. This is called ______.
For first time Private sector was given priority compared to the public sector in.
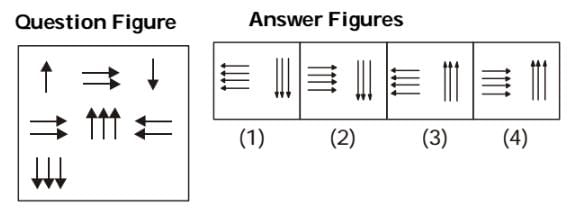
In the following question, which answer figure will complete the pattern in the question figure?

In a given number 7834329513, we interchange the first and the second digits, the third and the fourth digit and so on. Then which digit will be sixth from the right?
Identify the following:
1. - It is also referred to as a web address.
2. - It specifies the internet address of a file stored on a host computer or server connected to the internet.
Which of the following applications is used to create presentations?
The basic cause of a teacher`s failure in maintaining discipline is the lack of
What is the most effective way to discipline a mischievous student?
Effective teaching is by and large a function of teacher's
I− reduces IO3- and I2 and itself oxidised to I2 in acidic medium. Thus, final reaction is
Aniline does not undergo Friedel – Crafts reaction
Many copper (I) compounds are unstable in aqueous solution and undergo disproportionation as 2Cu+ → Cu + Cu2+ . This is due to
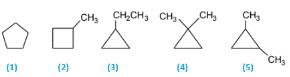
How many total cyclic isomers are possible for C5H10 ?
A metal crystallizes with a face-centered cubic lattice. The edge of the unit cell is 408 pm. The diameter of the metal atom is
Based on the following thermodynamic data,
Q. On the total mass basis of reactants, which reaction will generate the greatest amount of heat?
We have taken a saturated solution of AgBr.Ksp of AgBr is 12 x 10 – 14 . If 10 – 7 mole of AgNO3 are added to 1 litre of this solution then the conductivity of this solution in terms of 10 – 7 Sm – 1 units will be
[given 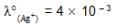 Sm2 mol-1
Sm2 mol-1 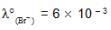 Sm2 mol-1, 5 x 10-3 Sm2mol-1]
Sm2 mol-1, 5 x 10-3 Sm2mol-1]
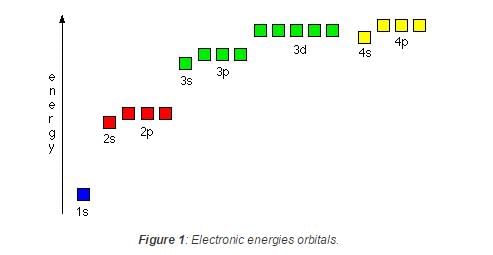
Which of the following sets of quantum numbers represents the highest energy of an atom ? [AIEEE 2007]
The increasing order of enthalpy of vaporization of NH3, PH3, and AsH3 is
Three elements needed for the healthy growth of plants are
Which of the following is thermally the most stable?
Schools should cater to individual differences to:
Determinants of individual differences in human beings relate to
Directions: Improve the bracketed part of the sentence.
Q. He (like) to picture himself as an original thinker.
Improve the bracketed part of the sentence with the parts given below.
Q. ONGC has claimed (to have start) a new updated natural gas refinery at Digboi.
In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.
Q. A man who is courteous and gallant in his behavior.
Directions: In the following question, one part of the sentence may have error(s). Find out the part of the sentence having an error and select the appropriate option. If a sentence is free from error, select 'No error' as your answer.
Q. You have been doing (1)/ your project work (2)/ routinely? (3)/ No error (4)
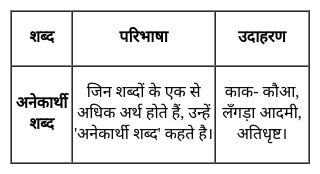
'अनादर, उपेक्षा, निरादर' के लिए कौन सा अनेकार्थी शब्द सही है चयन कीजिए?




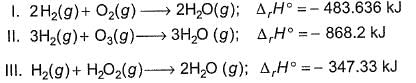 From I, total mass of reactant, 36 gm
From I, total mass of reactant, 36 gm