EMRS PGT Chemistry Mock Test - 6 - EMRS MCQ
30 Questions MCQ Test - EMRS PGT Chemistry Mock Test - 6
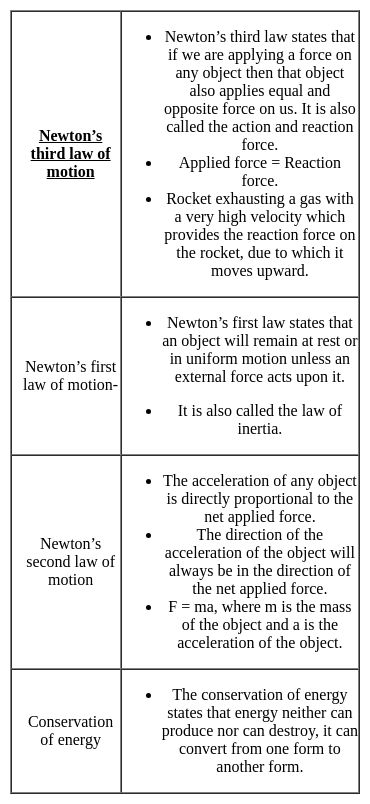
The working principle of a rocket engine is based on the-
A man walks 30 meters towards South, then turning to his right, he walks 30 meters, then turning to his left, he walks 20 meters, again turning to his left, he walks 30 meters. How far is he from his starting point ?
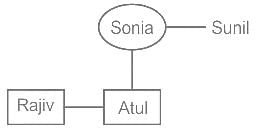
Rajiv is the brother of Atul. Sonia is the sister of Sunil. Atul is the son of Sonia. How is Rajiv related to Sonia?
Direction: Read the following information carefully and answer the questions that follow.
A blacksmith has five iron articles A, B, C, D and E each having a different weight.
I. A weight is twice as much as of B.
II. B weight is four and half times as much as of C.
III. C weight is half times as much as of D.
IV. D weight is half as much as of E.
V. E weight is less than A but more than C.
Q. E is lighter in weight than which of the other two articles?
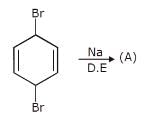
Which is the least reactive halide in both E1 and SN1 reaction?
When K2O is added to water, the solution becomes basic in nature because it contains a significant concentration of -
[Ti (H2O)6]3+ absorbs green and yellow region part of visible light. Then the transmitted colour of the compound is
90% of hydrogen peroxide is used as fuel in ______________
The most and least reactive electrophiles respectively in a SN1 reaction are
Which of the following forms a colloidal solution in water?
Direction:
This section contains 5 questions. When worked out will result in an integer from 0 to 9 {both inclusive).
Q.
Relative decrease in vapour pressure of an aqueous NaCI solution is 0.167. Thus, number of moles of NaCI present in 180 g of H20 is ... .
A mixture of ethyl alcohol and propyl alcohol has a vapour pressure of 290 mm at 300 K. The vapour pressure of propyl alcohol is 200 mm. If the mole fraction of ethyl alcohol is 0.6, its vapour pressure (in mm) at the same temperature will be -
[AIEEE 2007]
Elements of which group are known as ore forming elements?
What kind of support can a school provide to address the individual differences in students?
Which of the following is an example of scaffolding?
____is a mental health condition that involves extreme mood swings, including both manic and depressive episodes.
In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.
Q. A story that can be interpreted to reveal a hidden meaning.
In the following question, four words are given out of which one word is incorrectly spelled. Find the incorrectly spelled word.