EMRS PGT Chemistry Mock Test - 7 - EMRS MCQ
30 Questions MCQ Test EMRS PGT Mock Test Series 2025 - EMRS PGT Chemistry Mock Test - 7
The Right to Education (RTE) Act was enacted by the Parliament of India in the year _____.
Select the word-pair in which the two words are related in the same way as the two words in the following word-pair.
International Yoga Day : 21st June :: ? : ?
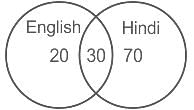
In a group of students, each one knows either Hindi or English. If 100 know Hindi, 50 know English and 30 know both, how many students are there in group?
The positions of how many digits in the number 837912 will remain unchanged after the digits within the number are rearranged in descending order (from left to right)?
Shalini investigates a topic thoroughly and does not need to be over-directed. In which learning phase is she?
The primary difference between the modern periodic table and Mendeleev's periodic table is:
A hydrogen like species in fourth orbit has radius 1.5 times that of Bohr's orbit. In neutral state, its valence electron is in
The standard enthalpies of formation of CO2(g), H2O (l) and glucose (s) at 25°C are - 400 kJ mol-1, - 300 kJ mol-1 and - 1300 kJ mol-1 respectively. The standard enthalpy of combustion per gram of glucose at 25°C is
[JEE Advanced 2013]
The pKa of a weak acid (HA) is 4.5. The pOH of an aqueous buffered solution of HA in which 50% of the acid is ionized is:
18 g of glucose (C6H12O6) is added to 178.2 g of water. The vapour pressure of water for this aqueous solution at 100º C is -
[AIEEE 2006]
The salt which finds uses in qualitative inorganic analysis is -?
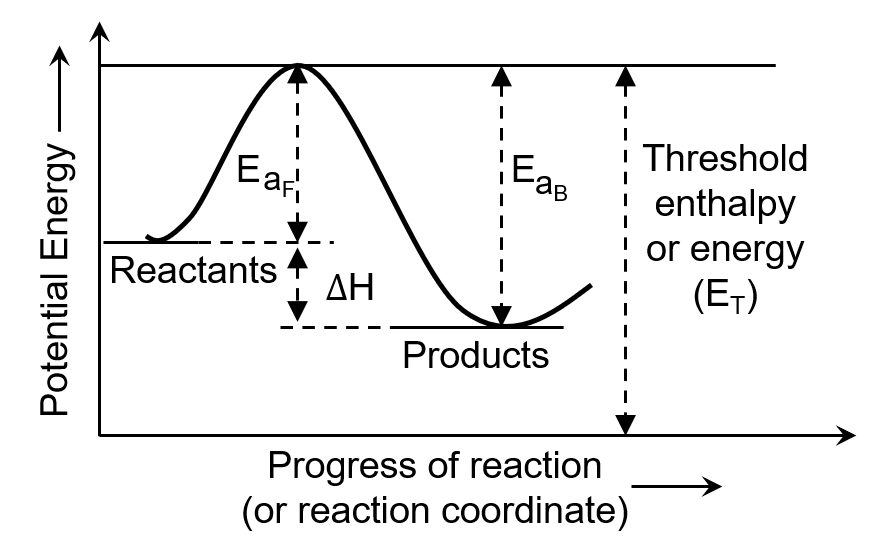
The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is called
For moderation of the climate and body temperature of living beings, the responsible factor is:
Direction (Q. Nos. 1-13) This section contains 13 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. If the enthalpy of HCI (g) and Cl- (aq) are - 92.30 kJ mol-1 and - 167.44 kJ mol-1 respectively, then ΔrH° for the following reaction is
HCI (g) + aq → H+ (aq) + Cl- (aq)
Ionisation energy of He+ is 19.6x10-18 J atom -1. The energy of the first stationary state (n = 1)of Li2+ is
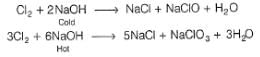
What product are formed during the electrolysis of a concentrated aqueous solution of sodium chloride using an electrolytic cell in which electrodes are separated by a porous pot?
I. Cl2(g)
II. NaOH(aq)
III. H2(g)
IV. NaClO(aq)
V. NaClO3(aq)
Select the correct choice.
Amongst the following compounds, the optically active alkane having lowest molecular mass is -
[AIEEE-2004]
At 298K the standard free energy of formation of H2O(L) is – 237.20kJ/mole while that of its ionisation into H+ ion and hydroxyl ions is 80 kJ/mole, then the emf of the following cell at 298 K will be
H2(g,1 bar) | H+ (1M) || OH– (1M) | O2 (g, 1bar)
According to Vygotsky why do children speak to themselves?
In the following question, an idiomatic expression and its four possible meanings are given. Find out the correct meaning of the idiom.
A drop in the bucket.
|
8 docs|45 tests
|




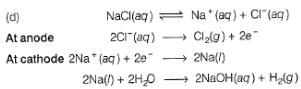

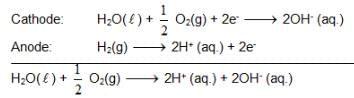 also we have
also we have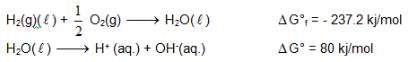 Hence for cell reaction
Hence for cell reaction