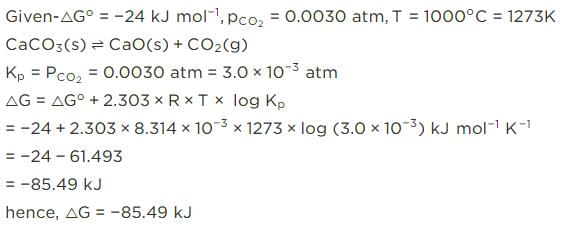EMRS PGT Chemistry Mock Test - 8 - EMRS MCQ
30 Questions MCQ Test - EMRS PGT Chemistry Mock Test - 8
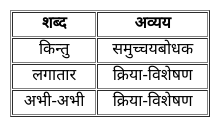
The new agricultural strategy called 'Green Revolution' was initiated in ________.
Which of the following is not a department under Ministry of Finance?
From the given alternatives, select the word which CANNOT be formed using the letters of the given word.
FEARLESS
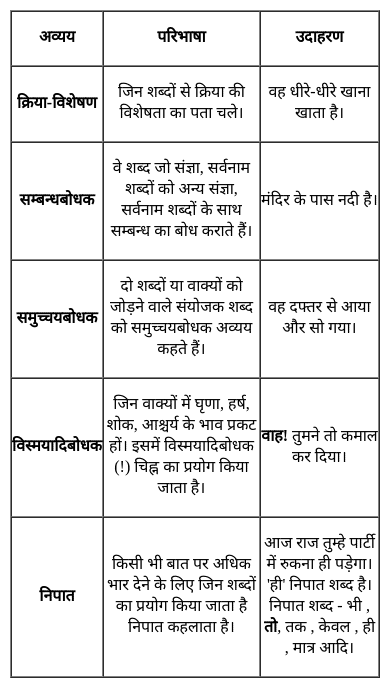
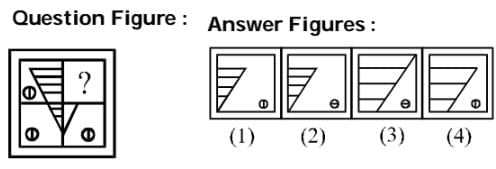
Which one of the answer figures shall complete the given question figure?
Picture formats can be recognized by which extensions?
What are the steps taken to open an existing document?
Which of the following is the most important factor underlying the success of a new teacher?
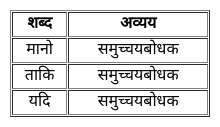
In which of the following pairs both the ions are coloured in aqueous solution? (Atomic number, Sc = 21, Ti = 22, Ni = 28, Cu = 29, Co = 27)
Direction (Q. Nos. 17-20) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Which of the following can be calculated based on Born-Haber cycle of formation of a lattice A+B- (s) from A(s) and 6 (g) ?
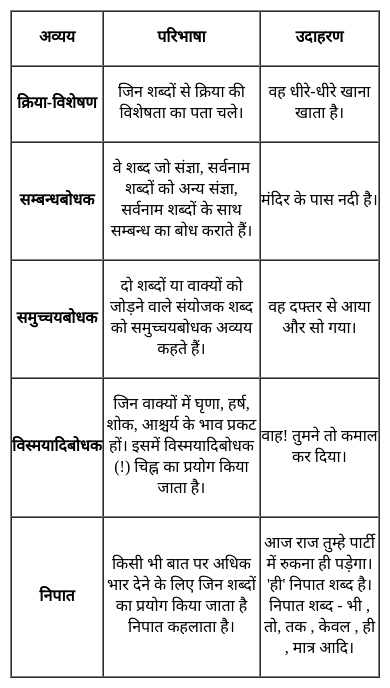
For the reaction, at 1000° C
ΔG° = - 24 kJ mol-1, 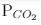 = 0.0030 atm.
= 0.0030 atm.
Q. Hence, ΔG at this temperature is
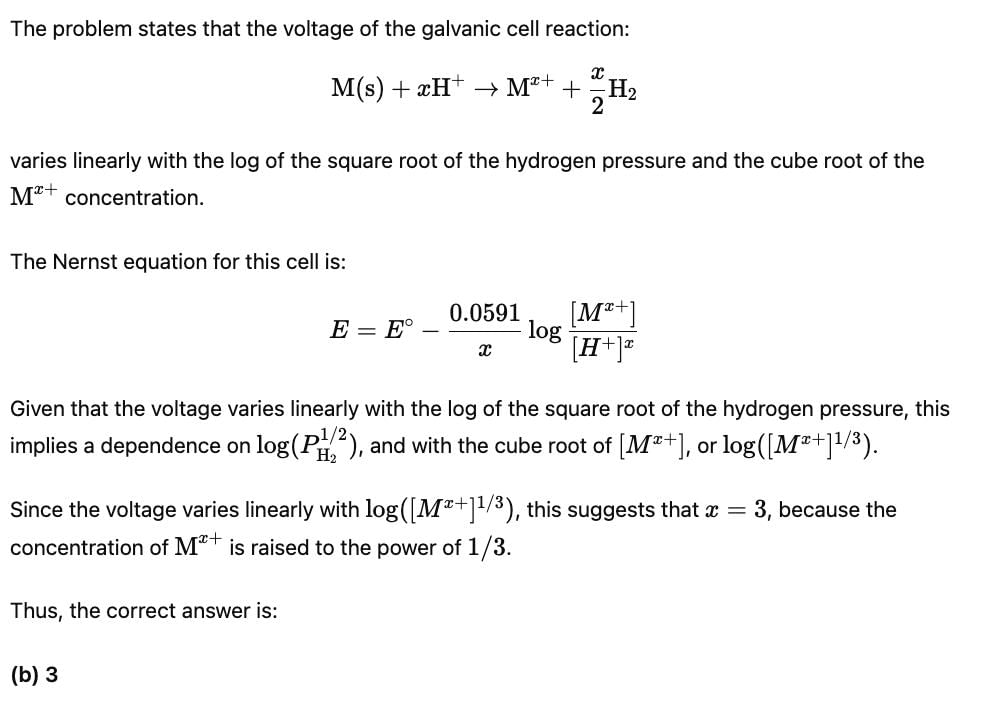
It is observed that the voltage of a galvanic cell using the reaction M(s) + xH+→ Mx+ + X/2H2 varies linearly with the log of the square root of the hydrogen pressure and the cube root of the Mx+ concentration. The value of x is
The alkali metals are low melting. Which of the following alkali metal is expected to melt if the room temperature rises to 30°C?
In an atom, an electron is moving with a speed of 600 m/s with an accuracy of 0.005%. Certainity with which the position of the electron can be located is (h = 6.6×10-34 kg m2s-1 ,mass of electron, em = 9.1×10-31 kg ) [AIEEE 2009]
A metal M readily forms water soluble sulphate, and water insoluble hydroxide M(OH)2. Its oxide MO is amphoteric, hard and having high melting point. The alkaline earth metal M must be -
Comprehension Type
Direction (Q. Nos. 16-18) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Consider the following sequence of reaction,
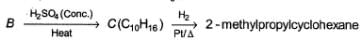
Q.
The structure of compound B is
In Calgon’s method, one of the following chemical is used to remove hardness of water
Which one of the following exists in the oxidation state other than +3?
H2 gas is adsorbed on the metal surface like tungsten. This follows........ order reaction –
[AIEEE-2002]
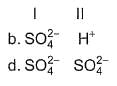
Consider the following reactions,
I. Zn + dil. H2SO4 → ZnSO4 + H2
II. Zn + conc. H2SO4 → ZnSO4+ SO2 + H2O
Oxidising agents in I and II are
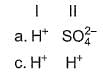
Directions: In the following question, one part of the sentence may have error(s). Find out the part of the sentence having an error and select the appropriate option. If a sentence is free from error, select 'No error' as your answer.
Q. The rich man (1)/ killed him (2)/ and his own children (3)/ No error (4)