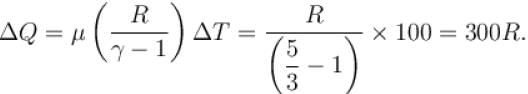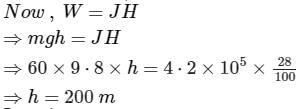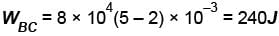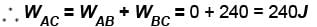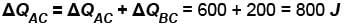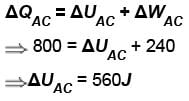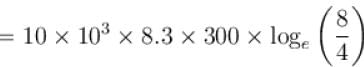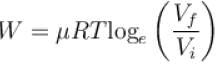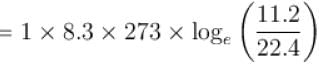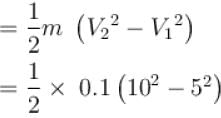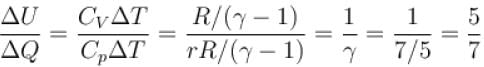First Law Of Thermodynamic NAT Level - 1 - IIT JAM MCQ
10 Questions MCQ Test - First Law Of Thermodynamic NAT Level - 1
If R = universal gas constant, the amount of heat needed to raise the temperature of 2 mole of an ideal monoatomic gas from 273K to 373K when no work is done (in units of R)
The weight of a person is 60kg. If he get 105 calories heat through food and the efficiency of his body is 28%, then up to how much height, he can climb (in meters)?
A thermodynamic process is shown in the figure. The pressure and volumes corresponding to some points in the figure are :
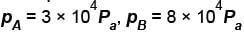
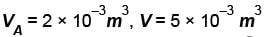
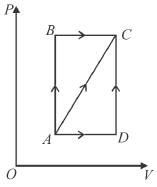
In process AB, 600 Joules of heat is added to the system and in process BC, 200J of heat is added to the system. The change in the internal energy of the system in process AC would be? (in Joules)
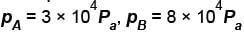
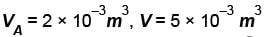

In process AB, 600 Joules of heat is added to the system and in process BC, 200J of heat is added to the system. The change in the internal energy of the system in process AC would be? (in Joules)
How much energy (in 107 Joules) is absorbed by 10kg molecule of an ideal gas if it expands from its initial pressure of 8 atm to 4 atm at a constant temperature of 27ºC.
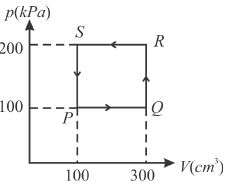
A thermodynamic system is taken through the cycle PQRSP process. The net work done by the system is (in Joule)
One mole of O2 gas having a volume equal to 22.4 liters at 0ºC and 1atm pressure is compressed isothermally so that its volume reduces to 11.2 liters. The work done in this process is (in Joules)?
A gas is compressed at a constant pressure of 50N/m2 from a volume of 10m3 to a volume of 4m3. Energy of 100J is then added to the gas by heating. Then increase in its internal energy is?
A block of mass 100gm slides on a rough horizontal surface. If the speed of the block decreases form 10m/s to 5m/s, then thermal energy developed in the process in Joules is?
When an ideal diatomic gas is heated at constant pressure, the fraction of the heat energy supplied which increases. the internal energy of the gas is?
If CV = 4.96 cal/mole-K, then increase in internal energy when temperature of 2moles of this gas is increased from 340K to 342K (in cal)?