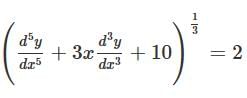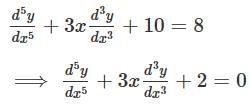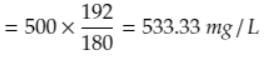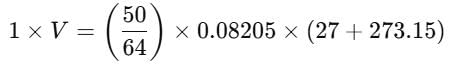GATE Environmental Science Mock Test - 3 - GATE Environmental Science MCQ
30 Questions MCQ Test GATE Environmental Science 2026 Mock Test Series - GATE Environmental Science Mock Test - 3
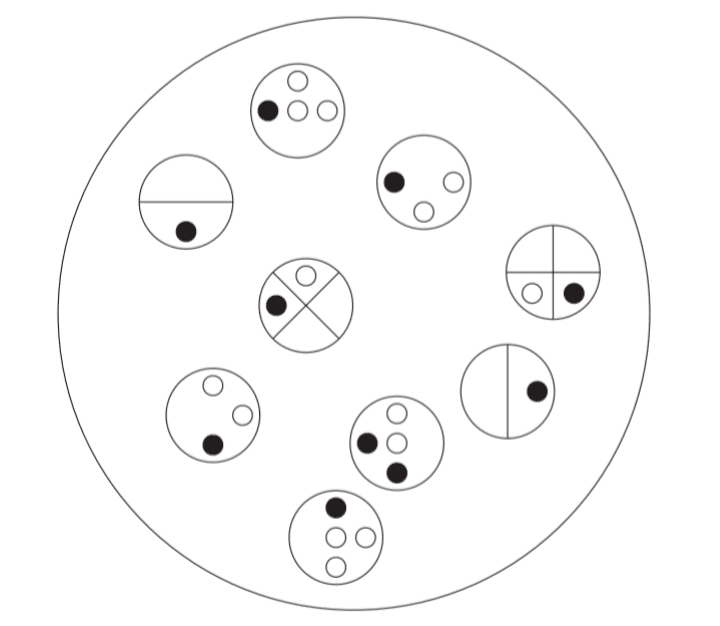
In the provided diagram, there are 15 sections that need to be colored in such a way that no two adjacent sections sharing a boundary (excluding corners) have the same color. What is the minimum number of colors necessary to achieve this?

The figure below displays three distinct perspectives of a dice.

Which piece of paper can be folded to form this dice?

Which piece of paper can be folded to form this dice?
Which of the following pieces of equipment for controlling air pollution is most effective for eliminating particles that are 15 microns in size?
Which of the following statements is incorrect regarding endospores?
The presence of hardness in water is NOT attributed to ____
The highest coordination number achievable by Sn4 is _____
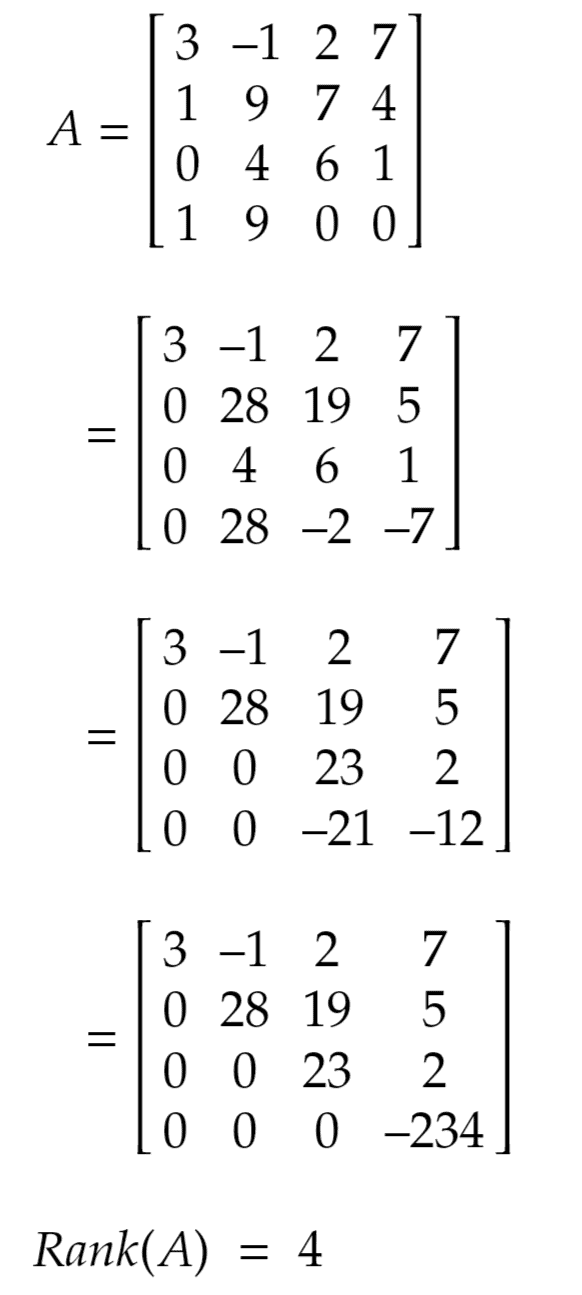
Determine the rank of the matrix shown below:
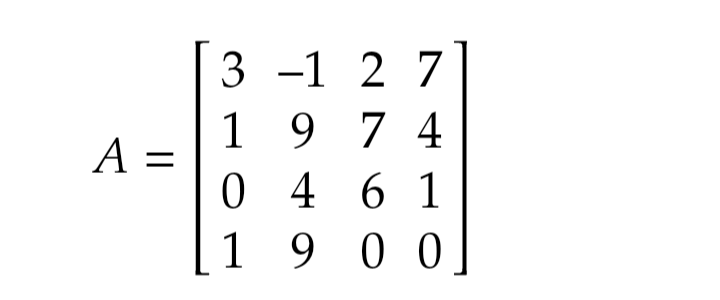
What is the degree of the differential equation shown below?
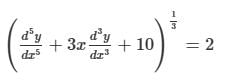
Bacterial cells that are rod-shaped are referred to as
The primary cause of tuberculosis is ______
The corrosion at the crown of reinforced cement concrete sewers is attributed to
Which of the following statements is accurate?
If X represents a vector, and A and B denote linear operators, what are the correct mathematical relationships that hold true?
The function f(x) = x 3 — 4.5 x 2 — 12x has a local maximum at x = _____ (an integer value) within the range x = 2 to 2.
Consider the differential equation dy/dx - x2 ex = 0, with the initial condition y = 1 when x = 0.
\nWhat is the value of y at x = 1 ____ (rounded to 2 decimal places)?
\nAssume the value of e (the base of natural logarithm) is 2.7.
Given that the solubility product (Ksp) of silver bromide is 5 x 10–9 at a temperature of 25 °C, determine its approximate solubility (in g/L, rounded to three decimal places). (Note: M.W of Ag = 108, Br = 80)
The theoretical oxygen demand for a glucose solution with a concentration of 500 mg/L (in mg/L, rounded to two decimal places) is ___.
In relation to the flexible mechanisms established by the Kyoto Protocol, which of the following statements is/are correct?
1. The Clean Development Mechanism (CDM) allows any Annex I country to invest in emission reduction initiatives in any other Annex I country as a substitute for making domestic emission reductions.
2. Through Joint Implementation (JI), countries can achieve their domestic emission reduction goals by acquiring greenhouse gas reduction units from projects located in non-Annex I countries to the Kyoto Protocol (primarily developing nations).
Select the correct option from the codes given below:
Organize the following elements of an Environmental Management System in the correct order:
1. Planning
2. Review
3. Implementation
4. Environmental Policy
5. Monitoring
Which of the following is/are categorized as hazardous waste?
Note: This is a MSQ. One or more than one option can be correct.
Which of the following statements is/are accurate?
Note: This is a MSQ. One or more options may be correct.
Consider the function f(x) = ln(sin(x)).
Expand f(x h) using Taylor's series. In this scenario, which statement(s) is/are correct?
Regarding disinfection, which of the following statement(s) is/are CORRECT?
The oxygen sag equation proposed by Streeter-Phelps for rivers is founded on several assumptions. Which of the following assumption(s) is/are correct?
You have two types of waste materials:
1. Sludge contains an arsenic concentration of 150 mg/kg (wet) and has a solid content of 20%
2. Wood Chips have an arsenic concentration of 500 mg/kg (wet) and a moisture content of 30%
If these components are combined in equal proportions by total weight, what will be the arsenic concentration in the dry sludge? (Expressed in mg/kg, rounded to the nearest integer)
The level of sulphur dioxide (SO2) in the surrounding atmosphere was recorded at 50 µg/m3. Under identical conditions, what is the concentration of SO2 represented in ppb, rounded to two decimal places?
Given: pressure = 1 atm, Temperature = 27 °C
The pH of a solution containing 0.1 M acetic acid and 0.05 M sodium acetate is ________ (rounded to 2 decimal places).
The pKa value for the ionization of acetic acid is 4.76.
The ionic strength of a solution that has 0.01 M of CaCl2 and 0.001 M of Na2SO4 is __________ M (rounded to three decimal places).
A wastewater treatment facility processes one million liters per day (MLD) of wastewater that has a soluble BOD of 200 mg/L. The BOD level of the effluent after treatment is 20 mg/L. The yield coefficient observed for the biological system is 0.35. Calculate the daily biomass production in the system in __________ kg (an integer value).