GATE Life Science Mock Test- 1 (Biochemistry & Micro Biology) - GATE Life Sciences MCQ
30 Questions MCQ Test GATE Life Sciences 2026 Mock Test Series - GATE Life Science Mock Test- 1 (Biochemistry & Micro Biology)
Which of the following is a synonym for the term DISPARAGE?
If θ represents the angle, in degrees, formed by the longest diagonal of a cube and any of the cube's edges, then what is the value of cosθ?
What is the total of the first n terms in the sequence 9 99 999 .... ?
A jigsaw puzzle consists of 2 pieces. One of the pieces is illustrated above. Which of the provided options for the missing piece, when combined, will create a rectangle? The piece can be moved, rotated, or flipped to connect with the above piece.

What is the geometry of Fe(CO)5?
(Note: Atomic number of Fe is 26)
The rate of a reaction involving A and B reduces to one-fourth when the concentration of B is increased twofold. What is the order of the reaction concerning B?
A solution containing a compound exhibits an absorbance of 0.42 at a wavelength of 275 nm in a cuvette with a light path of 0.1 dm. The molar absorptivity of this compound is ε275 = 8.4 × 103 M–1 cm–1. What is the concentration of the compound in ______× 10–5 M (rounded to the nearest integer)?
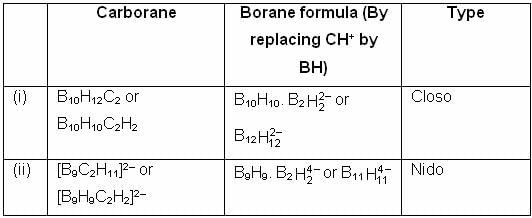
According to Wade's rule, the configurations of [B10C2H12] and [B9C2H11]2 – respectively are
What is the bond order of the O–O bond in the O22– ion?
A total of 4 g of NaOH is dissolved in volumes of 100 ml, 500 ml, 1000 cm3, and 1900 cm3 to create four stock solutions labeled A, B, C, and D. If 99 ml of a 1 M HCl solution is added, which of the stock solutions will have a pH that is closest to 7?
There are two crystalline varieties of lead oxide (PbO), commonly referred to as red oxide and yellow oxide. The standard enthalpy of formation (ΔH°f) for these oxides is – 219.0 kJ for the red form and – 217.3 kJ for the yellow form.
What is the value of ΔH° when the yellow form of oxide transitions to the red oxide through a solid-solid phase change?
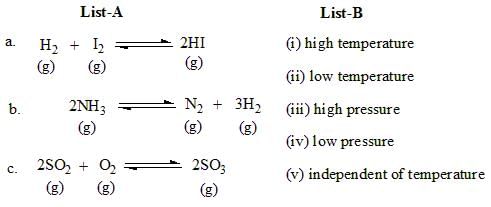
Pair the items in list - A, which represent reversible chemical reactions, with the corresponding conditions in list - B, and identify the correct answer based on the options provided below:
The VALID combination(s) of Y and T for the subsequent elimination reaction is(are)

Among the following options, which species is(are) diamagnetic?
(Given: Atomic numbers of Fe = 26, Co = 27, and Ni = 28)
The pKa value of the guanidinium group in Arginine is _____
Which antibiotic listed below has the ability to create an ion channel within the bacterial membrane?
Intracellular concentrations of ATP, ADP, and inorganic phosphate in four cell types are given below. Which one of these cell types has the most negative ∆G for ATP hydrolysis?

Which of the following amino acids possesses more than two acid-base groups?
Which of the following types of cells do not possess hypoxanthine-guanine phosphoribosyltransferase (HGPRT)?
Which of the following techniques can be employed to isolate proteins based on their molecular weights from a protein mixture?
A solution of 100 ml with a pH of 10 was thoroughly mixed with another 100 ml solution having a pH of 4. What is the pH of the resulting 200 ml solution, rounded to two decimal places?
The classical method of Gram staining is ineffective for Nocardia spp. due to the presence of
Which of the following statements regarding bacterial flagella is accurate?
Which of the following enzymes catalyze(s) the process of substrate-level phosphorylation?
A culture of photosynthetic green algae was exposed to 14CO2 light for a brief period. The initial metabolite in the Calvin cycle that will be radiolabeled is
A mutation that acts as a nonsense suppressor is identified in ________.
Pair the classes of Immunoglobulin with their respective functions.

Which of the following genera are classified as spirochetes?
Which of the following inclusion bodies are non-membrane bound?
When Escherichia coli is cultivated in optimal conditions, it undergoes division every 20 minutes. Given an initial population of 100 Escherichia coli cells, what will the logarithmic count of cells be at the 17th generation? (Round your answer to one decimal place)