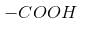HPSC PGT Chemistry Mock Test - 1 - HPSC TGT/PGT MCQ
30 Questions MCQ Test HPSC PGT Mock Test Series 2025 - HPSC PGT Chemistry Mock Test - 1
Who called the Brahma Sarovar of Haryana as a miniature sea?
Suppose you are teaching at the primary level. You often see that the students give a response which is correct to a certain extent. In such case, what would be your duty as a teacher?
Electronic configuration of the element having atomic number 24.
The element with lowest ionization energy among the following is:
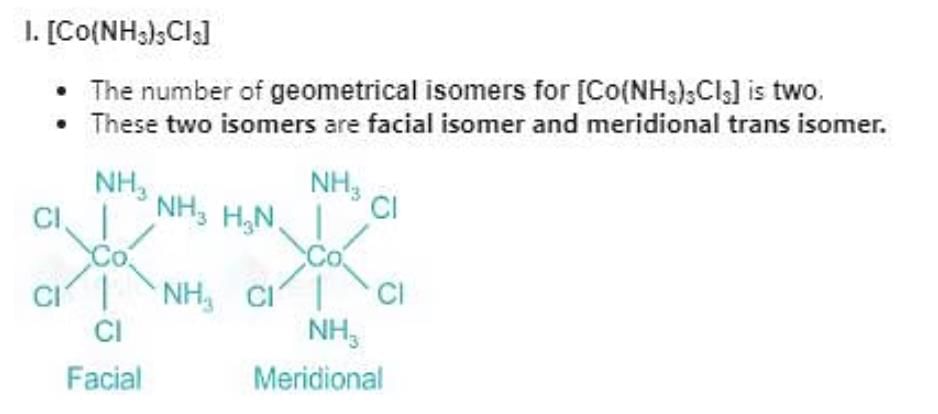
Which of the following compounds will show facial and meridional isomerism?
(A)[Co(NH3)3Cl3]
(B)[Co(acac)3]
(C)[Co(dien)(NO2)3]
(D)[Co(gly)3]
Only One Option Correct Type
This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
CsCI crystallises in a cube that has CP at each corner and Cs+ at the centre of the unit cell. If rCs+ = 169 pm and rcl- =181 pm, then edge length of the cube is
Only One Option Correct Type
Direction (Q. Nos. 1- 13) This section contains 13 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Consider the following reaction,
Q.
the major product in the above reaction is
Out of the following, amphiprotic species are
I : HPO32-
II OH-
III H2PO4-
IV HCO3-
A vessel of 250 litre was filled with 0.01 mole of Sb2S3 and 0.01 mole of H2 to attain the equilibrium at 440°c as
Sb2S3(s) + 3H2(g) 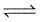 2Sb(s) + 3H2S(g).
2Sb(s) + 3H2S(g).
After equilibrium the H2S formed was analysed by dissolving it in water and treating with excess of Pb2+ to give. 1.195 g of PbS (Molecular weight = 239) precipitate.
What is value of Kc of the reaction at 440°C ?
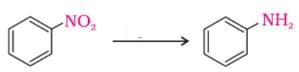
The following reaction takes place in the presence of
Direction (Q. Nos. 1 - 6) This section contains 6 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
A pure enantiomer with molecular formula C6H13OBr, when reacted with PBr3, an achiral product C6H12Br2 is obtained that has no chiral carbon. The compound which satisfy this condition could be (no bond to chiral carbon is broken during the reaction)
1 Faraday of electricity is passed through the solution containing 1 mole each of AgNO3, CuSO4, AlCl3 and SiCl4. Elements are discharged at the cathode.Number of moles of Ag, Cu,Al and Si formed will be in the ratio of
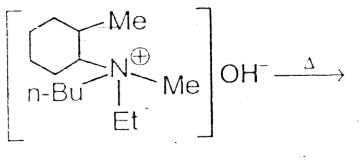
The alkene formed as a major product in the above elimination reaction is
[AIEEE 2006]
In the structure of NaCI given below, ratio rNa+/rcl- is
Direction (Q, Nos. 1 - 5) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which molecule will give the following dicarboxylic acid upon treatment with acidic solution of KMnO4?
Direction (Q. Nos. 1-15) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. For the
I. benzene (C6H6) and
II. borazine (B3N3H6)
Select the correct statement.
Arrange the following compounds in decreasing order of their acid strength: i) trichloroacetic acid ii) trifluoroacetic acid iii) acetic acid and iv) formic acid
The electronegativity of following elements increases in the order of?
Which of the following species does not show disproportionation reaction?
The enthalpy of neutralisation of HS- (aq) is - 5.1 kJ mol-1. Thus, second ionisation energy of H2S is
Direction (Q. Nos. 1-12) This section contains 12 multiple-choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Consider the following reactions.
I. Zn + dil. H2So4 → ZnSO4 + H2
II. Zn + conc. H2SO4 → ZnSO4 + SO4 + H2O
III. Zn + HNO3 → Zn(NO3)2 + H2O + NH4NO3
Q. Oxidation number of H changes in:



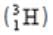 has 1 proton and 2 neutrons.
has 1 proton and 2 neutrons. 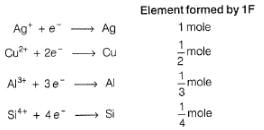

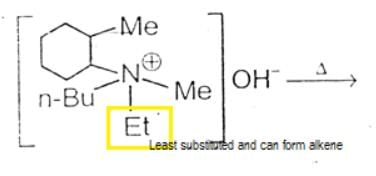


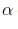 - position.
- position.