HPSC PGT Chemistry Mock Test - 10 - HPSC TGT/PGT MCQ
30 Questions MCQ Test HPSC PGT Mock Test Series 2025 - HPSC PGT Chemistry Mock Test - 10
In Haryana which of the following is not comprised in Ahir Women’s dress?
The lowest region of atmosphere in which the human being along with other organisms live is known as:
Alkaline earth metals combine with halogens to form:
When diazonium salt solution is treated with water at a temperature of 283 K it forms?
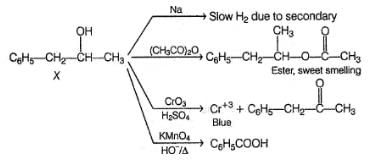
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
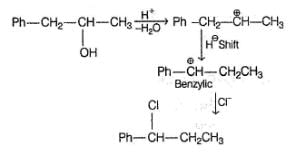
If X is treated with HCI in the presence of ZnCI2, the major product would be
What is the major product in the following reaction?
Direction (Q. Nos. 1- 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. For the given reaction, = - 1.3818 kcal at 300 K. Thus equilibrium constant is
2-Hexyne gives trans -2- Hexene on treatment with -
[AIEEE 2012]
1 mole N2 and 3 mol H2 are placed in a closed container at a pressure of 4 atm. The pressure falls to 3 atm at the same temperature when the following equilibrium is attained.
N2(g) + 3H2(g)  2NH3(g). The equilibrium constant Kp for dissociation of NH3 is :
2NH3(g). The equilibrium constant Kp for dissociation of NH3 is :
Requirement of macronutrient per acre of the land is
Direction (Q. Nos. 14 -15) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d).
Based on the following thermochemical data of the given process, answer the questions.
Q. Bond energy of (C— C) bond is
Which of the following molecular species has unpaired electron(s) ?
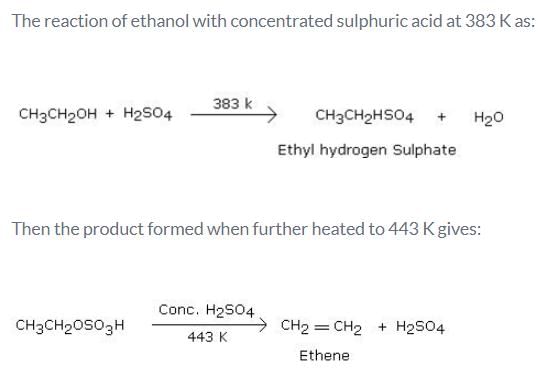
What is the product formed when excess of ethanol reacts with concentrated sulphuric acid at 383 K and then the temperature of reaction mixture is increased to 443 K?
Direction (Q. Nos. 1-16) This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. The correct statement for the molecule Csl-3 is
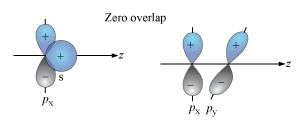
Which of the following orbitals give a zero overlap?
Covalent nature of NaF, Na2O and Na3N in the increasing order is
Water has a maximum density at _____ degree centigrade.
When Al2O3 is electrolysed ,cation and anions are discharged. For a given quantity of electricity,ratio of number of moles of Al and O2 gas is
For the following equilibrium starting with 2 moles SO2 and 1 mole O2 in 1 L flask,
Equilibrium mixture required 0.4 mole in acidic medium. Hence, Kc is