HPSC PGT Chemistry Mock Test - 2 - HPSC TGT/PGT MCQ
30 Questions MCQ Test HPSC PGT Mock Test Series 2025 - HPSC PGT Chemistry Mock Test - 2
The length of a rectangle is three-fifth of the radius of a circle. The radius of the circle is equal to the side of a square, whose area is 6400 m2. The perimeter (in m) of the rectangle, if the breadth is 15 m, is:
As per the NEP 2020, the Rajasthan government has decided to come up with a new syllabus that includes 3 languages, which are-
An alkaline earth metal nitrate on thermal decomposition yields:
If X is a nonmetal, its oxide X2O3 is expected to be a/an ______ oxide.
Which property is represented by the following figure;
Which of the following ligand gives chelate complexes?
Correct decreasing order of dipole moment of CH3F, CH3CI and CH3Br is
‘Hartree’ is the atomic unit of energy and is equal to
Which scientist proposed that atomic number is more fundamental property of an element than its atomic mass?
Consider the following transformations.
Q. Which reaction sequence will bent bring about the above transformation?
For the reversible reaction,
In a reaction vessel, [NO]= [O2]= 0.01 mol L-1 and [NO2]= 0.1 mol L-1 then above reaction is
Out of NH3, H2O and HF, which has the highest magnitude of Hydrogen bonding:
Consider the following set of molecules.
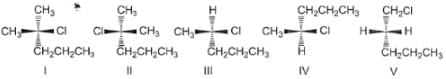
The pairs of enantiomers are
The ratio of bond pairs and lone pairs in a P4 molecule is
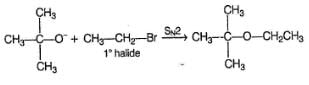
Which is the best reaction for preparation of t-butyl ethyl ether?
Substances that alter the rate of a chemical reaction without being used up in a chemical reaction is known as
A chemical reaction [2A] + [2B] + [C] → product follows the rate equation : then order of reaction is - [AIEEE-2002]
Which has lower standard reduction potential (SRP) value?
The molal elevation constant is the elevation in boiling point of
When body temperature is high, doctors advice consumption of light food. This is because
In the complete combustion of C2H6, 54 g of H2O is formed and 370 kcal of heat is evolved. Thus, ΔCH° of C2H6 is
In balancing the half-reaction, CN- → CNO-
The number of electrons that must be added is
If a is the degree of dissociation of Na2SO4, the vant Hoff's factor (i) used for calculating the molecular mass is–
[AIEEE-2005]
Select the set of compounds having fractional oxidation number in one or more atoms.