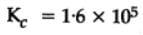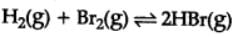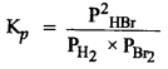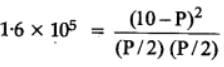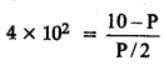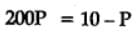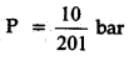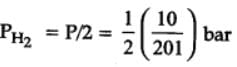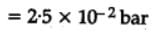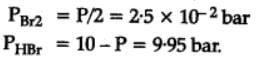HPSC PGT Chemistry Mock Test - 3 - HPSC TGT/PGT MCQ
30 Questions MCQ Test - HPSC PGT Chemistry Mock Test - 3
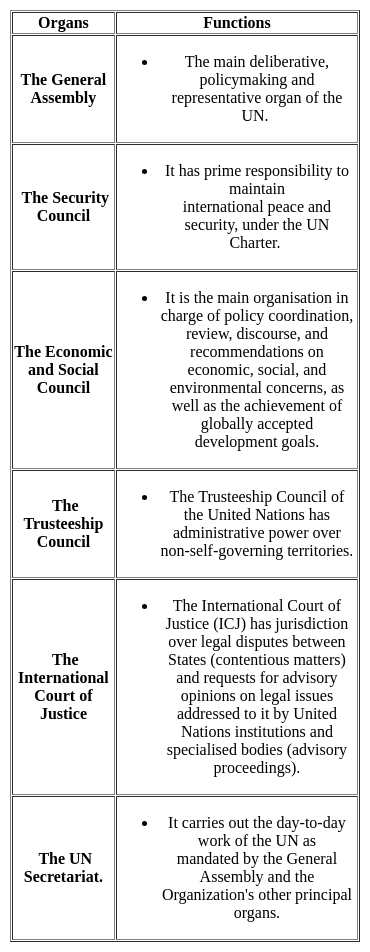
How many main organs are there in United Nations Organization?
Who among the following has been appointed as the first ADGP Traffic of Haryana recently?
How can teachers facilitate understanding of complex concepts in children?
Under NEP 2020, what is the target for the Gross Enrollment Ratio (GER) in higher education by 2035?
One kg of carbon produces __________ kg of carbon dioxide.
Which of the following compounds will exhibit cis-trans isomerism?
Passage I
A constant current of 30 A is passed through an aqueous solution of NaCl for a time of 1.00 h.
Thus Cl2 formed under STP condition is
Aerated water contains CO2 dissolved in water
CO2(g) + H2O(l)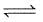 H2CO3(aq)
H2CO3(aq)
Variation of solubility (s) with pressure (p) is shown by
According to Bohr's theory, the angular momentum of an electron in 5th orbit is - [AIEEE 2006]
Among the following statements the one that is not true about Mendeleev’s Periodic Table is:
Ferric hydroxide is a negative sol, which of the following electrolyte will coagulate it most:
The golden yellow colour associated with NaCl to Bunsen flame can be explained on the basis of -
Adsorption isobar is a curve showing variation of adsorption ______.
In the modern periodic table, which period contains 32 elements?
Volume occupied by one molecule of water (density = 1 g cm-3) is
The equilibrium constant for the following reaction, is 1.6 x 105 at 1024 K.
H2(g) + Br2(g) ⇌2HBr(g)
HBr (g)at 10.0 bar is introduced into a sealed container at 1024 K. Thus, partial pressure of H2(g)and Br2(g), together is
Pick out the most reactive alkyl halide for an SN1 reaction.
Match the Column I with Column II and mark the correct option from the codes given below.
Passage I
An unknown compound X (C5HgBr) does not decolourise purple colour of alkaline permanganate solution. Upon treatment with C2H5OK/C2H5OH, X gives Y (C5H8) as only structural isomer. Catalytic hydrogenation of Y gives methyl cyclobutane. Ozonolysis of Y gives Z (C5H8O2) which can exist as a pair of enantiomers.
Q.
What is true regarding reaction of Y with cold, dilute alkaline KMnO4 solution?
What is relative reactivity of secondary versus primary hydrogens in free radical bromination of n-butane if the ratio of 1-bromo to 2-bromobutane formed is 7 : 39?
Ca(HCO3)2 is strongly heated and after equilibrium is attained, temperature changed to 25° C.
Ca(HCO3)2(s)⇌CaO(s) + 2CO2 (g) + H2O(g)
Kp = 36 (pressure taken in atm)
Thus, pressure set up due to CO2 is