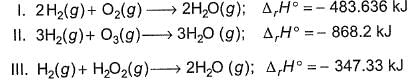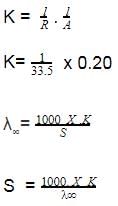HPSC PGT Chemistry Mock Test - 4 - HPSC TGT/PGT MCQ
30 Questions MCQ Test - HPSC PGT Chemistry Mock Test - 4
Identify the incorrect statement among the following.
Solubility of s block halides in water depends upon:
A gas absorbs 200 J of heat and expands by 500 cm3 against a constant pressure of 2 x 105 Nm-2. Change in internal energy is
The catalytic activity of transition metals and their compounds is mainly due to
Which is the most suitable reagent for the following transformation?
The nature of bonds in the compounds of carbon is mostly:
The value of log10 K for a reaction, A B is (Given, ΔH°298 = - 54.07 kJ mol-1;
ΔS°298 = + 10 JK-1 mol-1; R = 8.314 JK-1 mol-1 2.303 x 8.314 x 298 = 5705)
[IITJEE2007]
Racemic mixture is obtained due to the halogenation of
Which substance is added to water containing suspended impurities to coagulate the suspended impurities and make water fit for drinking purposes.
Which of the following species has tetrahedral geometry?
In the equation Kt = log C0 – log Ct, the curve between t and log Ct is -
[AIEEE-2002]
The half life of a reaction is halved as the initial concentration of the reactant is doubled. The order of the reaction is
Which of the following statements is true about the following conformer (Y)?
Based on the following thermodynamic data,
Q. Which oxidising agent will generate the greatest amount of energy per gram of oxidising agent?
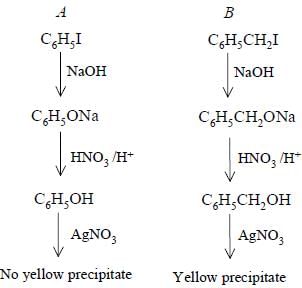
Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.
Which one of the following statements is true for this experiment ?
[AIEEE-2003]
The solubility of [Co(NH3)4Cl2] CIO4_________ if the = 50,
= 70, and the measured resistance was 33.5Ω in a cell with cell constant of 0.20 is ____.
Which of the following gaseous molecule is monoatomic?
Stoichiometric compounds of dihydrogen are formed with
The correct statement regarding elements of symmetry and chirality of compound is
Consider the following oxidation/reduction process,
Q. Magnetic moment does not change in
Passage II
A solution containing 10 g of a dibasic acid in 1000 g of water freezes at -0.15° C. 10 mL of the acid is neutralised by 12 mL of 0.1 N NaOH solution. [Kf (H20 ) = 1.86° mol-1 kg]
Q.
van’t Hoff factor (/') of the dibasic acid is
Which of the follownig compounds is (S)-4-chloro-1-methylcyclohexene ?
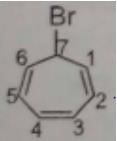
The number of cis-trans isomer possible for the following compound:
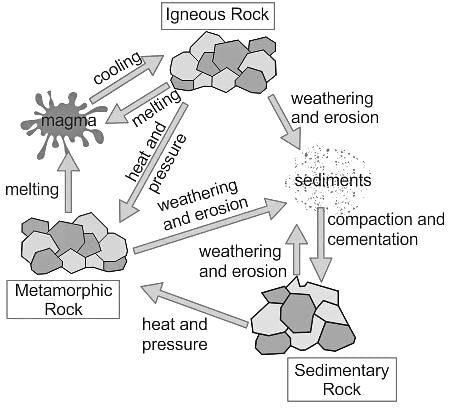
Passing carbon dioxide through slaked lime gives: