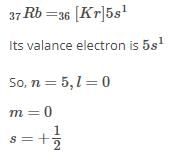HPSC PGT Chemistry Mock Test - 6 - HPSC TGT/PGT MCQ
30 Questions MCQ Test - HPSC PGT Chemistry Mock Test - 6
The stench due to leakage from LPG cylinder because of :
Mahatma Gandhi was first arrested in which railway station of Haryana?
A word with letters jumbled has been given. Choose the correct order of letters which are required to form the correct word.
Jumbled word: PTIOISCL
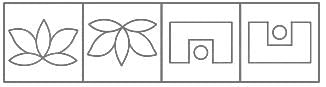
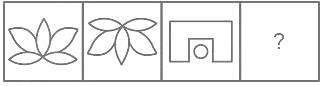
Which figure from the answer figures will replace the question mark (?) in the problem figures?
Problem Figures:
Answer Figures:
Amit is twice as good as Ramit. Working together they can complete the work in 28 days. In how many days can Ramit alone can do the same whole work?
For the reaction system: 2NO(g) + O2(g) → 2NO2(g) volume is suddenly reduced to half its value by increasing the pressure on it. If the reaction is of first order with respect to O2 and second order with repect to NO, the rate of reaction will –
[AIEEE-2003]
A hydrogen electrodes is immersed in a solution with pH = 0 (HCl). By how much will the potential (reduction) change if an equivalent amount of NaOH is added to the solution. (Take PH2 = 1 atm) T = 298 K.
C—Cl bond in (vinyl chloride) is stabilised in the same way as in
Which physical quantity remain unaffected when a reversible reaction is carried out in presence of a catalyst
Thermal decomposition of a compound is of first order. If 50% of a sample of a compound is decomposed in 120 min, the time taken for 90% completion is
Which of the following forms a colloidal solution in water?
For the reaction,
2SO2 (g) + O2 (g)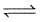 2SO3 (g) + 188.3 KJ
2SO3 (g) + 188.3 KJ
the number of moles of SO3 formed is increased if
Chlorine, bromine and iodine when combined with oxygen, have oxidation numbers
Among the following the element with highest first ionization energy is:
Which of the following equation depicts reducing nature of H2O2?
Noble gases are inert and do not form compounds like other elements because of their:
Number of atoms is 560 g of Fe (atomic mass = 56 g mol-1)
How many atoms of hydrogen are in 67.2 L of H2 at STP?
A mixture of ethyl alcohol and propyl alcohol has a vapour pressure of 290 mm at 300 K. The vapour pressure of propyl alcohol is 200 mm. If the mole fraction of ethyl alcohol is 0.6, its vapour pressure (in mm) at the same temperature will be -
[AIEEE 2007]
When K2O is added to water, the solution becomes basic in nature because it contains a significant concentration of -
The correct set of four quantum numbers for the valence electron of rubidium atom (Z = 37) is
[JEE Main 2013]
Chemical species present in the environment are either naturally occurring or generated by human activities. Their interrelation with the surroundings is called:
Direction (Q. Nos. 11-14) This section contains 2 paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Passage l
Sulphur undergoes a phase transition between 80 and 110°C
S(rhombic) S (monoclinic); ΔH° = 3.213 kJ mol-1; ΔS° = 8.71 JK-1 mol-1
Q. Select the correct alternate(s).
The constant k used in rate equation is known as
Which of the following statements are not true for hydrogen?