HPSC PGT Chemistry Mock Test - 7 - HPSC TGT/PGT MCQ
30 Questions MCQ Test - HPSC PGT Chemistry Mock Test - 7
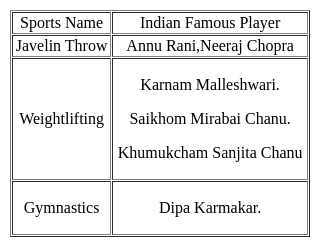
Haryana government has announced to build a huge sports stadium in the village of which famous sportsperson?
According to NEP 2020, what is the role of teachers in the new education system?
The basis of selection of a teaching aid is
- age of the learner
- objectives of teaching
- intellectual level of the learner
Choose from the options given below:
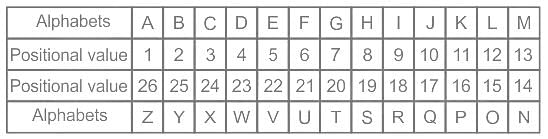
Where would you find the smallest atoms of elements in the periodic table?
What does hydrogen peroxide liberate from potassium iodide?
At what temperature white phosphorous changes to red phosphorous?
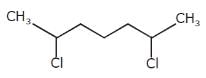
What is the major bromination product in the following reaction?
The substance that gets adsorbed on the surface of the solid is called?
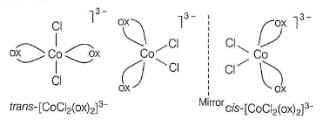
Which of the following will have three stereoisomeric form ?
i. [Cr(NO3)3(NH3)3]
ii. K3[Co(C2O4)3]
iii. K3[Co(C2O4)2CI2]
iv. [Co(en)2CIBr]
How many structural isomers are possible with molecular formula C4H10O ?
In the following compounds, select the points of interm olecular H-bonding (linear),
One of the following rubbers is used in making oil seals, tank lining, etc.
The reagent which can best bring about the following transformation is
Direction (Q. Nos. 7 - 10) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
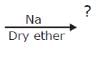
Q. Predict product(s) in the following reaction.
(Yellow ppt) T X
Y. (Yellow ppt) + Z (pungent smellinggas)
If X gives green flame test. Then, X is :
The standard enthalpies of formation of CO2(g), H2O (l) and glucose (s) at 25°C are - 400 kJ mol-1, - 300 kJ mol-1 and - 1300 kJ mol-1 respectively. The standard enthalpy of combustion per gram of glucose at 25°C is
[JEE Advanced 2013]
The primary difference between the modern periodic table and Mendeleev's periodic table is:
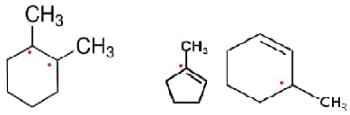
How many assymmetric carbon atoms are present in
(i) 2-Dimethyl cyclohexane
(ii) 3-Methyl cyclopentene
(iii) 3-Methylcyclohexene
Atmosphere traps the sun’s heat near the earth’s surface. This is called:
In a 0.2 molal aqueous solution of a weak acid HX the degree of ionization is 0.3 . Taking kƒ for water as 1.85, the freezing point of the solution will be nearest to _
[AIEEE-2003]
When an electron is added to neutral gaseous atom to convert it into a negative ion, the enthalpy change accompanying the process is called: