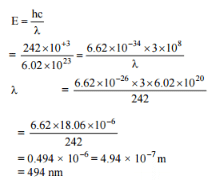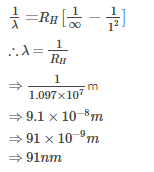HPSC PGT Chemistry Mock Test - 9 - HPSC TGT/PGT MCQ
30 Questions MCQ Test HPSC PGT Mock Test Series 2025 - HPSC PGT Chemistry Mock Test - 9
The average pattern of wind speed, temperature, rainfall etc., in place over a long period of time is called ___________.
Devi Shankar Prabhakar is associated with which of the following field?
At which place of Haryana, women participated in a movement which was started by daughters of Lala Duli Chand, namely, Vidhyawati, Yashoda and Jamuna?
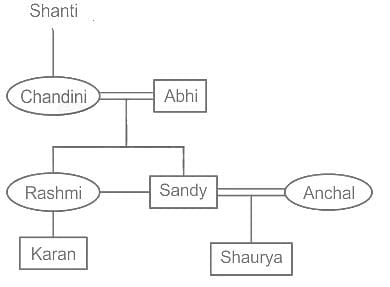
Shanti’s daughter Chandini is married to Abhi. Anchal is married to Sandy, the grandson of Shanti. Abhi's grandson is Karan. Rashmi is the mother of Karan. Shaurya is Anchal's son. How is Shaurya related to Karan?
Choose the alternative which is an odd word/number/letter pair out of the given alternatives.
Name the gas whose formula was established by Sorret?
Direction (Q. Nos. 23 and 24) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q.
Match the species in Column I with the structure in Column II.
In the catalyzed decomposition of benzene diazonium chloride,
Half life period is found to be independent of the initial concentration of the reactant. After 10 min, the volume of N2 gas collected is 10 L and after the reaction is complete, it is 50 L. Hence, the rate constant of the reaction(in min-1) is
The maximum number of atomic orbitals associated with a principal quantum number 5 is
What is the correct structure for the major compound produced by the following reaction sequence?
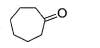
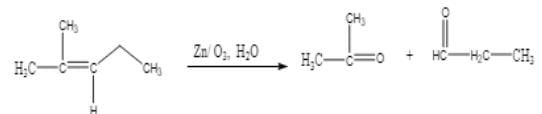
Ozonolysis of an organic compound ‘A’ produces acetone and propionaldehyde in equimolar mixture. Identify ‘A’ from the following compounds :
[AIEEE 2011]
The energy required to break one mole of Cl - Cl bonds in Cl2 is 242 kJ mol-1. The longest wavelength of light capable of breaking a single Cl - Cl bond is(c = 3 x 108 ms-1 and NA = 6.02 x 1023 mol-1) [AIEEE 2010]
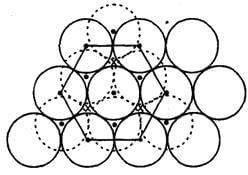
In hexagonal close packing of sphere in three dimensions.
What type of interaction hold the molecules together in a polar molecular solid?
Direction (Q. Nos. 11-14) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. For an ideal gas, consider only (p -V) work in going from initial state X to the final state Z. The final state Z can be reached either of the two paths shown in the figure. Which of the following choice (s) is (are) correct?
(Take ΔS as change in entropy and W as work done)
[IIT JEE 2012]
Sulfide ion in alkaline solution reacts with solid sulfur to form polysulfide ions having formulas S22-, S32-, S42- and so on. The equilibrium constant for the formation of S22- is 12 (K1) & for the formation of S32- is 132 (K2), both from S and S2-. What is the equilibrium constant for the formation of S32- from S22- and S
What volume of hydrogen gas at 273 K and 1 atm pressure will be consumed in obtaining 21.6 g elemental boron (atomic mass = 10.8) from the reduction of boron trichloride by hydrogen?
Comprehension Type
Direction (Q. Nos. 12-14) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Consider the following reaction. The tertiary butyl group can be cis or trans to tosylate group. Answer the following questions based on the knowledge of E2 and E1 reaction mechanism.
Q.
The correct statement concerning above elimination reaction is
Reaction
+ HOCl → product,
here product will be -
[AIEEE-2002]
We know that the relationship between Kc and Kp is Kp = Kc (RT)Δn
What would be the value of Δn for the reaction NH4Cl (s) ⇔ NH3 (g) + HCl (g)
The wavelength of the radiation emitted, when in a hydrogen atom electron falls from infinity to stationary state 1, would be (Rydberg constant = 1.097×107 m-1) [AIEEE- 2004]
Newland arranged elements in increasing order of atomic weights and noted that every eighth element had properties similar to:
In the electrosynthesis,potassium manganate (VII) is converted to manganese(IV) dioxide. By passage of 1F of electrolysis ,one mole of potassium manganate(VII) will form manganese dioxide.
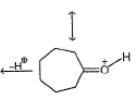
NO2 can be represented as
Q. Formal charge on each oxygen atom is
Which of the following statements about photochemical smog is wrong?