IIT JAM Chemistry - MCQ Test 2 - Chemistry MCQ
30 Questions MCQ Test - IIT JAM Chemistry - MCQ Test 2
The order of decreasing energy among covalent bond (CB), dipole-dipole interaction (DDI) and London dispersion forces (LDF) is:
T50 (half-life period) of first order reaction is 10 min. Starting with 10 mol L-1, rate after 20 min is:
The set representing the correct order of ionic radius is:
Which of the following magnetic moment values will correspond to highest ionization energy form Mn species:
For the reaction 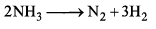
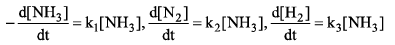 Then relation between k1, k2 and k3 is
Then relation between k1, k2 and k3 is
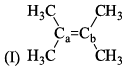
Arrange the following compounds in order of Ca—Cb bond length:
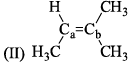
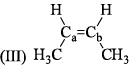
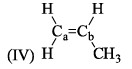
All the following are the resonance structure of one another except:
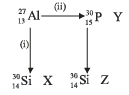
Bombardment of aluminium by α-particle leads to its artificial disintegration in two ways (i) and (ii) as shown. Product X, Y and Z respectively are:
Heat of dissociation of CH3COOH is 0.005 kcal g-1, hence enthalpy change when 1 mole of Ca(OH)2 is completely neutralized by CH3COOH is:
The heat released on neutralization of CsOH with all strong acids is 13.4 kcal mol-1. The heat released on neutralization of CsOH with HF (weak acid) is 16.4 kcal mol-1. ΔH° of ionization of HF in water is:
At low pressure, that Vander Waal’s equation is reduced to:
Correct order of stability of the following carbocation is:
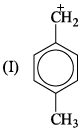
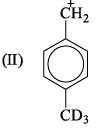

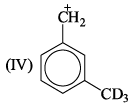
If 3/4 quantity of a radioactive element disintegrates in two hours its half- life would be:
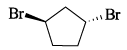
What is the stereochemical relationship between the following compound:
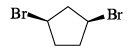
Among the structures given below, the most stable conformation for the following compound is:
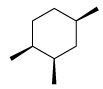
Following configuration of the tartaric acid represents:
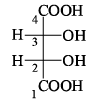
The gauche conformation (θ = 60°) of n-butane possess:
Rate of formation of SO3 in the following reaction
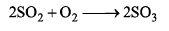
is 100g min-1. Hence, rate of disappearance of O2 is:
Any gas shows maximum deviation from ideal gas at:
The de-Broglie wavelength of the electron in the ground state of hydrogen atom is ? Given: KE = 13.6 eV, 1 eV = 1.6*10 -19J.
For a first order homogenous gaseous reaction, 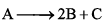 then initial pressure was P, while total pressure after time ‘t’ was Pt. The write expression for the rate constants k in terms of Pi, Pt and t is:
then initial pressure was P, while total pressure after time ‘t’ was Pt. The write expression for the rate constants k in terms of Pi, Pt and t is:
In which of the following reactions, hydrogen peroxide acts as on oxidizing agent:
Heat of neutralization of H2C2O4 (oxalic acid) is - 2 6 kcal mol-1. Hence dissociation energy of:
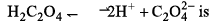
Which is the correct order of dipole moments of the compounds:
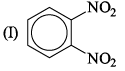
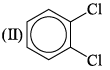
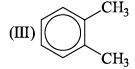
The correct order of increasing bond angles in the following triatomic species
The schrodinger wave equation for hydrogen atom is:
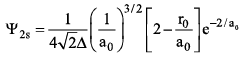
where ao is Bohr radius. Find position of radial node.