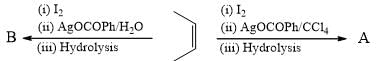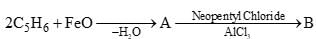IIT JAM Chemistry Mock Test 1 - Chemistry MCQ
30 Questions MCQ Test - IIT JAM Chemistry Mock Test 1
For the following equilibrium, N2O4(g) → 2NO2(g) Kp is found to be equal to Kc. This is attained when:
Aluminium-25 decay by emitting a positron. The species immediately produced has:
The temperature coefficient of two reactions are 2 and 3 respectively. Which would be correct for these reactions?
Arrange the following in decreasing order of stretching frequency 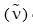 of C-O bond (cm–1):
of C-O bond (cm–1):
(I) Mo(CO)3(NMe3)3 (II) Mo(CO)3[P(OPh)3]3
(III) Mo(CO)3(PMe3)3 (IV) Mo(CO)3(PCl3)3
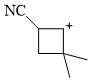
Which of the following form stable for CN—CH2—CH2—CN?
By Cannizaro reaction A changes to B and C as given. Hence, A is:
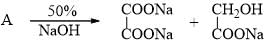
Emf of Cd-cell is 1.018 V at 25°C. The temperature coefficient of cell is –5.2 × 10–5 VK–1. How cell temperature will change during operation?
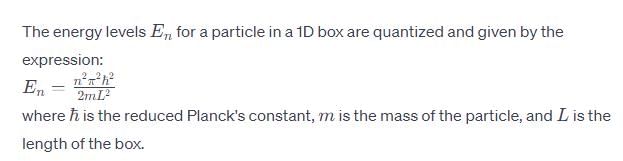
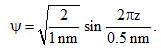 Calculate En if
Calculate En if 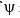 represents state of a particle in 1-D box of length 1nm:
represents state of a particle in 1-D box of length 1nm:
The self-ionization constant for pure formic, acid, has been estimated as 10–6 at room temperature. The density of formic acid is 1.15 g/cm3. The percentage of formic acid molecules in pure formic acid are converted to formate ion:
has been estimated as 10–6 at room temperature. The density of formic acid is 1.15 g/cm3. The percentage of formic acid molecules in pure formic acid are converted to formate ion:
25 mL of hydrogen and 18 mL of Iodine when heated in closed container, produced 30.8 mL of HI at equilibrium. Calculate degree of dissociation of HI at same temperature:
For a surface inactive substance (excessive concentration of solute per unit area of surface) is essentially:
Which of the following does not show facial & meridonial isomerism?
The plane(s) which show allowed reflection in both bcc and fcc lattices is/are:
(I) 100 (II) 111 (III) 110
Consider μ1, μ2, μ3 are dipole moments of Pyrrole, Furan, Thiopene respectively. Which of the following holds true:
Which of the following conversion take place in bourdon tubes?
Which of the following is true about Osazone formation in carbohydrates?
If bond length of CO bond in carbon monoxide is 1.128 Å, then, what is the value of CO bond length in Fe(CO)5?
Consider borax. Chemical formula of which is Na2B4O7. 10H2O. Choose the incorrect statement:
Which of the following will give different product on hydrolysis (among others)?
1H NMR spectrum of a compound with molecular formula C4H9NO2 shows:
d 5.30 (broad, 1H); d 4.10 (q, 2H); d 2.80 (d, 3H); d 1.20 (t, 3H). The structure of compound that is consistence with the above data is:
If sin2q = 0.1198, 0.2395, 0.3588, 0.4793, 0.5984. Find lattice type:
Which of the following conversions is/are correctly presented?


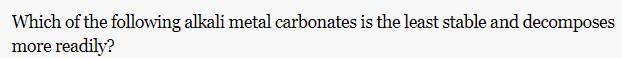




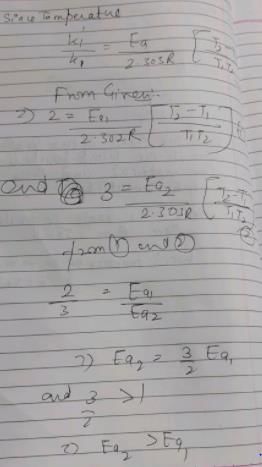
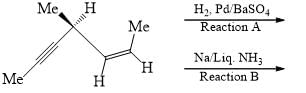
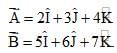
 Which of the following holds true?
Which of the following holds true?