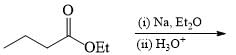IIT JAM Chemistry Mock Test 3 - Chemistry MCQ
30 Questions MCQ Test Mock Test Series for IIT JAM Chemistry - IIT JAM Chemistry Mock Test 3
With respect to electrophilic aromatic substitution, reactivity order of pyrrole, pyridine, furan and indole is:
Red color complex ion formed on adding FeCl3 to sodium extract, when N and S both are present in organic compounds is:
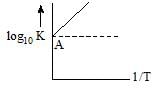
Vairation of log10 K with1/T is shown by the following graph in which straight line is at 45°, hence ∆H° is:
How many sigma and pi bonds are present in tetracyanoethylene?
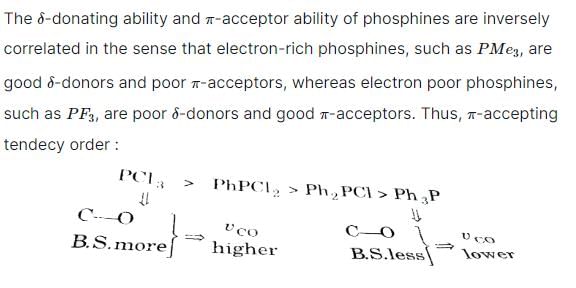
Arrange the following complex in increadsing order of stretching vibrational frequency of C-O bond :
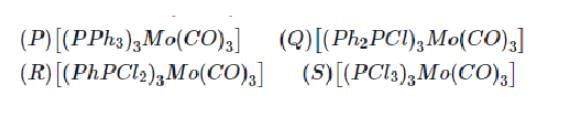
The process of removing lighter gangue particles by washing in a current of water is called:
Low oxidation state complexes are often air-sensitive, but are rarely water sensitive because:
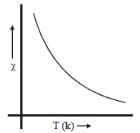
Plot of magnetic susceptibility with temperature show variation as given below. Such behaviour corresponds to:
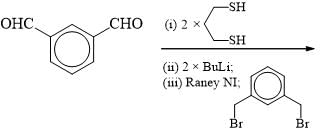
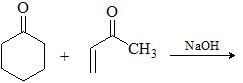
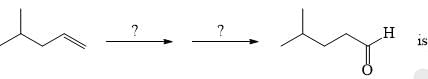
The suitable reaction steps for the following transformation:
Consider following reagent:
(I) Br2 water (II) Tollen's reagent (III) Fehling's solution
Which can be used to make distinction between an aldose and a ketone?
For a particle with projection r = 2i – 3j + k and momentum p = i + 2j – 2k in meter and Kg m/sec respectively, calculate the magnitude of angular momentum:
If the radius of first Bohr orbit be ao, then the radius of third orbit would be:
In the following radioactive decay, 232X92 → 220Y89. How many a and β- particles are ejected from X to form Y?
If excluded volume is taken zero, compressibility factor Z is
At a certain temperature vapour pressure of pure ether is 646 mm and that of pure acetone is 283 mm. Calculate the mole fraction of each component in the vapour state if the mole fraction of ether in the solution is 0.50?
Compounds K2Ba[Cu(NO2)6] (A) and Cs2Ba[Cu(NO2)6] (B) exhibit tetragonal elongation and tetragonal compression, respectively. The unpaired electron in A and B are found respectively, in orbitals:
The correct order of energy level of d-orbital or ferrocene is:
Calculate ΔH at 358 K for the reaction 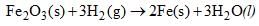
Given that DH298 = – 33.29 kJ mole–1 and Cp for Fe2O3(S), Fe(s), H2O(l) and H2(g) are 103.8, 25.1, 75.3 and 28.8 J/k mole respectively.
The virial expansion for a real gas can be written in either of the following forms:
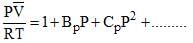
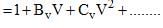
If Bv = aBp, the value of a would be
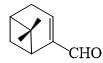
In the 1H NMR spectrum of myrtenal, the two methyl groups are expected to display signals at (chemical shift values (δ) m ppm)
|
2 docs|25 tests
|