JEE Exam > JEE Tests > Mock Tests for JEE Main and Advanced 2025 > JEE Main Chemistry Test- 3 - JEE MCQ
JEE Main Chemistry Test- 3 - JEE MCQ
Test Description
25 Questions MCQ Test Mock Tests for JEE Main and Advanced 2025 - JEE Main Chemistry Test- 3
JEE Main Chemistry Test- 3 for JEE 2025 is part of Mock Tests for JEE Main and Advanced 2025 preparation. The JEE Main Chemistry Test- 3 questions and answers have been
prepared according to the JEE exam syllabus.The JEE Main Chemistry Test- 3 MCQs are made for JEE 2025 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for JEE Main Chemistry Test- 3 below.
Solutions of JEE Main Chemistry Test- 3 questions in English are available as part of our Mock Tests for JEE Main and Advanced 2025 for JEE & JEE Main Chemistry Test- 3 solutions in
Hindi for Mock Tests for JEE Main and Advanced 2025 course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt JEE Main Chemistry Test- 3 | 25 questions in 60 minutes | Mock test for JEE preparation | Free important questions MCQ to study Mock Tests for JEE Main and Advanced 2025 for JEE Exam | Download free PDF with solutions
Detailed Solution for JEE Main Chemistry Test- 3 - Question 1
JEE Main Chemistry Test- 3 - Question 3
The nature of inter-molecular forces among benzene molecules is
Detailed Solution for JEE Main Chemistry Test- 3 - Question 3
JEE Main Chemistry Test- 3 - Question 5
The addition of HCN to a carbonyl compound is an example of
Detailed Solution for JEE Main Chemistry Test- 3 - Question 5
JEE Main Chemistry Test- 3 - Question 7
In the presence of platinum catalyst, hydrocarbon A adds hydrogen to form n-hexane. When hydrogen bromide is added to A instead of hydrogen, only a single bromo compound is formed. Which of the following is A ?
JEE Main Chemistry Test- 3 - Question 10
The alkene 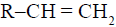 reacts readily with
reacts readily with 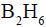 and formed the product B which on oxidation with alkaline hydrogen peroxides produces
and formed the product B which on oxidation with alkaline hydrogen peroxides produces
JEE Main Chemistry Test- 3 - Question 12
Amongst the following, the compound that can be most readily sulphonated is
Detailed Solution for JEE Main Chemistry Test- 3 - Question 12
Detailed Solution for JEE Main Chemistry Test- 3 - Question 14
JEE Main Chemistry Test- 3 - Question 15
Which of the following compound will make precipitate most readily with 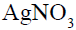 ?
?
JEE Main Chemistry Test- 3 - Question 16
Which of the following is responsible for depletion of the ozone layer in the upper strata of the atmosphere?
JEE Main Chemistry Test- 3 - Question 17
7.5 grams of a gas occupy 5.6 litres of volume at STP. The gas is
JEE Main Chemistry Test- 3 - Question 18
The number of water molecules present in a drop of water (volume 0.0018 ml) at room temperature is
Detailed Solution for JEE Main Chemistry Test- 3 - Question 18
JEE Main Chemistry Test- 3 - Question 19
The correct representation of Charle’s law is given by
JEE Main Chemistry Test- 3 - Question 20
The structure of TlCl is similar to CsCl. What would be the radius ratio in TlCl ?
*Answer can only contain numeric values
JEE Main Chemistry Test- 3 - Question 21
Sulphur trioxide is prepared by the following two reactions:-
S8(s) + 8 O2(g) —→ 8 SO2(g)
2SO2(g) + O2(g) —→ 2 SO3(g)
How many grams of SO3 are produced from 1 mole S8?
Detailed Solution for JEE Main Chemistry Test- 3 - Question 21
*Answer can only contain numeric values
JEE Main Chemistry Test- 3 - Question 22
How many elements are more electropositive than Cl?
Be, F, O, S, P, Au, H, Na
Detailed Solution for JEE Main Chemistry Test- 3 - Question 22
*Answer can only contain numeric values
JEE Main Chemistry Test- 3 - Question 23
How many elements have more ionisation energy as compared to their next higher atomic number element?
Na, Mg, Al, Si, P, S, Cl, Ar
Detailed Solution for JEE Main Chemistry Test- 3 - Question 23
*Answer can only contain numeric values
JEE Main Chemistry Test- 3 - Question 24
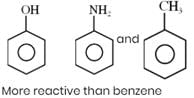
How many of the following compounds are more reactive than benzene towards electrophilic substitution.
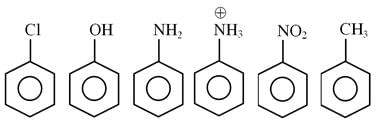
Detailed Solution for JEE Main Chemistry Test- 3 - Question 24
*Answer can only contain numeric values
JEE Main Chemistry Test- 3 - Question 25
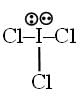
Number of d-orbitals used in the hybridisation of ICl3 is = x and number of lone pair at central atom = y find x + y = ?
Detailed Solution for JEE Main Chemistry Test- 3 - Question 25
|
356 docs|142 tests
|
Information about JEE Main Chemistry Test- 3 Page
In this test you can find the Exam questions for JEE Main Chemistry Test- 3 solved & explained in the simplest way possible.
Besides giving Questions and answers for JEE Main Chemistry Test- 3, EduRev gives you an ample number of Online tests for practice