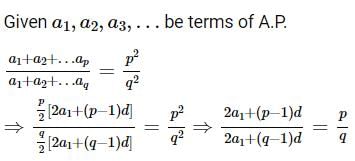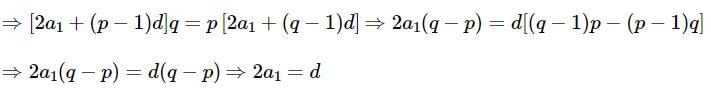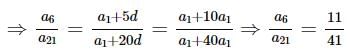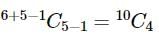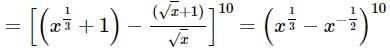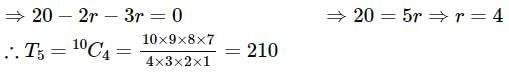JEE Main Mock Test - 7 - JEE MCQ
30 Questions MCQ Test Mock Tests for JEE Main and Advanced 2025 - JEE Main Mock Test - 7
A small coin is placed at a distance r from the centre of a gramophone record. The rotational speed of the record is gradually increased. If the coefficient of friction between the coin and the record is μ, the minimum angular frequency of the record for which the coin will fly off is given by
The ends of a stretched wire of length L are fixed at x = 0 and x = L. In one experiment, the displacement of the wire is y1 = A sin(πx/L) sin ωt and the energy is E1, and in another experiment the displacement is y2 = A sin(2πx / L)sin2ωt and the energy is E2. Then
A circular loop of wire 4 cm in radius carries a current of 80 A. Find the energy density at the centre of the loop.
A convex lens of focal length 10 cm is painted black at middle portion as shown in figure. An object is placed ar distance of 20 cm from the lens.
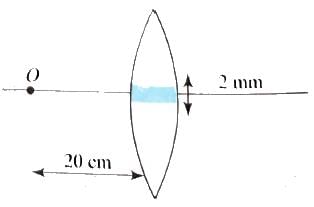
Then,
Assertion (A) When a wire of aluminium and another wire of silicon are heated from room temperature to 80∘C, the conductivity of aluminium increases and that of silicon decreases.
Reason (R) Aluminium has positive temperature coefficient of resistivity and silicon has negative temperature coefficient of resistivity.
Assertion: If light continuously falls on a metal surface, then the emission of electrons will stop after some time.
Reason: We cannot extract all the electrons of a metal.
A box is floating in the river of speed 5m/s. The position of the block is shown in the figure at t = 0. A stone is thrown from point O at time t = 0 with a velocity 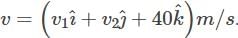 Find the value of v1, v2 such that the stone hits the box.
Find the value of v1, v2 such that the stone hits the box.

Two cannon ball A and B fall in water (density of balls is 0.6 x 103 Kg/m3 from the height 10m and 25m respectively. Find the ratio of the depth of ball A and B sink. Assume g=10m/s2
.
A light of intensity I0 is incident on a Polaroid which placed at 30º . Find the ratio of the intensity of light after and before polarization, if the light is polarized when Polaroid is at 60º.
A particle of mass 200 MeV/c2 collides with a hydrogen atom at rest. Soon after the collision, the particle comes to rest, and the atom recoils and goes to its first excited state. The initial kinetic energy of the particle (in eV) is N/4 . The value of N is (given the mass of the hydrogen atom to be 1 GeV/c2) ___________. (in integers)
An acid-base indicator has a Ka of 3.0 × 10−5. The acid form of the indicator is red and the basic form is blue. Then:
How much charge should be supplied to a cell for the electrolytic production of 245 gm NaClO4 from NaClO3 if the anode efficiency for the required reaction is 60% ?
Select the number of correct statements from the following:
(i) Delocalisation of σ electron is hyperconjugation
(ii) Delocalisation of π-electron is resonance
(iii) Inductive effect involves partial transfer of σ. electrons
(iv) Inductive effect involves partial displacement of σ elections
(v) Resonance effect is distance dependent
(vi) sp3, sp2 and sp hybridised carbon atoms can participate in resonance
Identify the correct statements from the following :
I. The ionic radius of Pr3+,Dy3+ and Sm3+ follow the order, Sm3+ > Pr3+ > Dy3+.
II. Eu2+ acts as strong reducing reagent.
III. Pu exhibits +7 oxidation state.
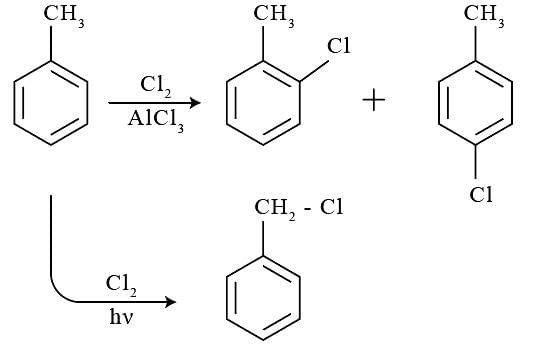
Statement-1: If chlorine is passed into toluene at room temperature in the presence of Anhydrous AlCl3, electrophilic substitution reaction takes place giving o- and p-chlorotoluenes.
Statement-2: If chlorine is passed through boiling toluene in the presence of UV light, substitution reaction in the aliphatic side chain occurs.
What is the main factor responsible for weak acidic nature of B-F bonds in BF3?
A line AB in three-dimensional geometry makes angles 45° and 120° with the positive x - axis and the positive y-axis respectively. If AB makes an acute angle e with the positive z -axis, then e equals:
Let z1,z2 be two complex numbers represented by points on the circle |z| =1 and |z| = 2 respectively then
The complete solution of the equation 7cos2x + sinx cosx − 3 = 0 is given by
The domain of function f(x) = loge(x − [x]) is (where [x] is greatest integer ≤ x)
A is a 3 × 3 matrix with entries from the set {−1, 0, 1}. Then the probability that A is neither symmetric nor skew-symmetric is
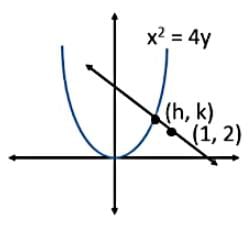
Find the equation of the normal at a point on the curve x2 = 4y which passes through the point (1.2)
In a shop, there are five types of ice-creams available. A child buys six ice-creams.
Statement-1: The number of different ways the child can buy the six ice-creams is 10C5.
Statement-2: The number of different ways the child can buy the six ice-creams is equal to the number of different ways of arranging 6 A's and 4 B's in a row.
The coefficient of the term independent of x in the expansion of 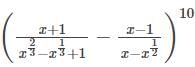 is
is
|
356 docs|142 tests
|


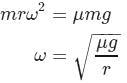

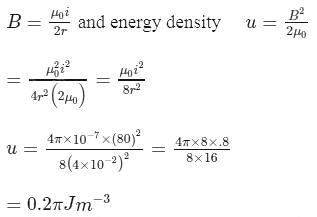
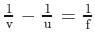
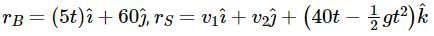
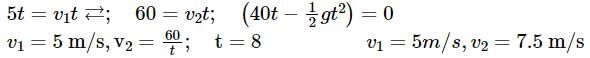
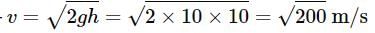
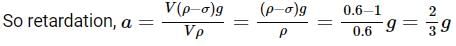

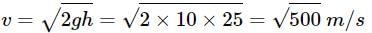
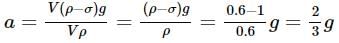

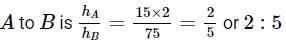
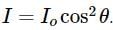 Since the light is polarized at angle 60º so the intensity of polarized light is
Since the light is polarized at angle 60º so the intensity of polarized light is 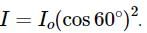
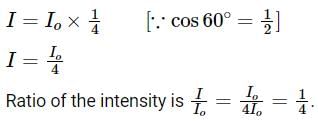
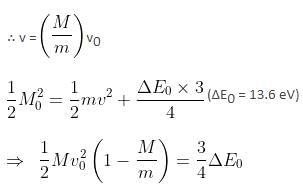
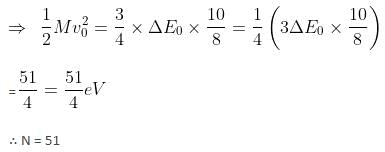
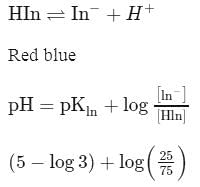
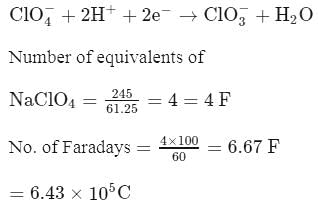
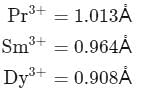
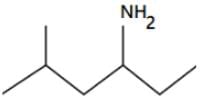
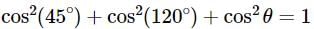
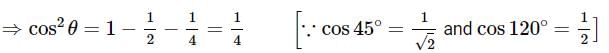
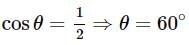

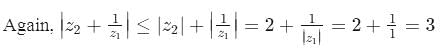
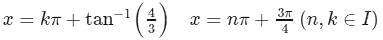
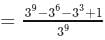
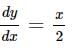
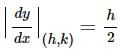
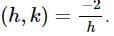
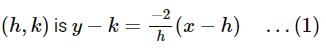
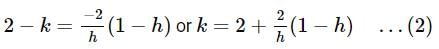
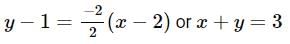
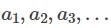 be terms of an A.P If
be terms of an A.P If 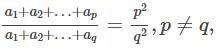 then
then 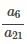 equals:
equals: