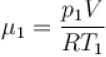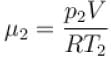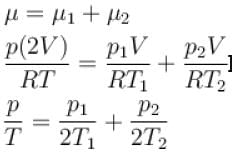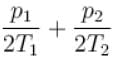Kinetic Theory Of Gases MCQ Level – 1 (part - 2) - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Kinetic Theory Of Gases MCQ Level – 1 (part - 2)
The pressure P, volume V and temperature T of a gas in the jar A and other gas in the jar B at Pressure 2P, volume V/4 and temperature 2T, then the ratio of number of molecules in jar A and B will be.
Select one:
Select one:
The equation of state corresponding to 8gm of O2 is.
Select one:
Select one:
The expansion of an ideal gas of mass m at a constant pressure P is given by the straight line D. Then the expansion of the same ideal gas of mass 2m at a pressure P/2 is given by the straight line.
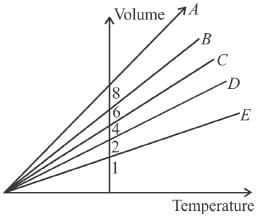
Select one:

Select one:
At room temp. (300K), the rms speed of the molecules of certain diatomic gas is found to be 1930 m/s. The gas is
Select one:
Root mean square speed of the molecules of ideal gas is v. If pressure is increased two times at constant temperature, the rms speed will become:
At top of a mountain, a thermometer reads 7°C and a barometer reads 70cm, of Hg. At the bottom of the mountain, these read 27°C and 76cm of Hg respectively.
Comparison of density of air at the top with that of bottom is.
Select one:
An experiment is carried on a fixed amount of gas at different temperature at high pressure such that it deviates from the ideal gas behavior. The variation of with p is shown in the diagram. The correct variation will correspond to.
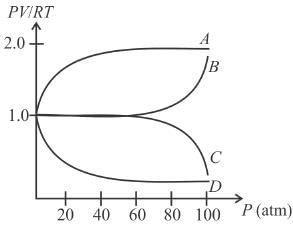
Select one:
Two containers of equal volume contain the same gas at pressure p1 and p2 and absolute temperature T1 and T2 respectively. On joining the vessels, the gas reaches a common pressure p and common temperature T. The ratio P/T is equal
to.
Select one:
The temperature of a gas is raised while its volume remains constant, the pressure exerted by a gas on the walls of the container increases because its molecules.
Select one:
The conversion of ideal gas into solids is.
Select one:


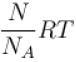 (N = Number of molecules, NA = Avogadro number)
(N = Number of molecules, NA = Avogadro number)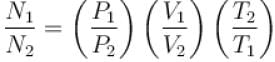
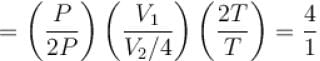
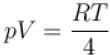

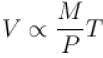
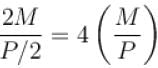 i.e., slope becomes four times so graph A is correct
i.e., slope becomes four times so graph A is correct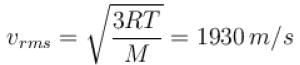
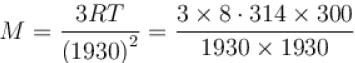
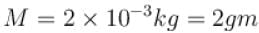
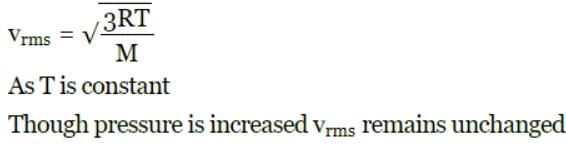
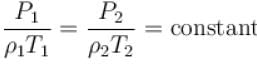
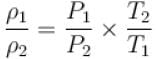
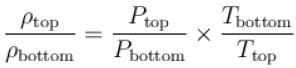
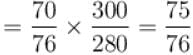
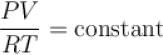 but when pressure increase, the decrease in volume will not take place in same proportion so PV/RT will increase.
but when pressure increase, the decrease in volume will not take place in same proportion so PV/RT will increase.