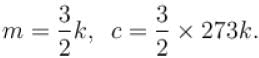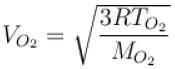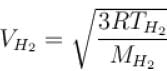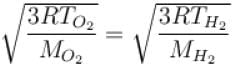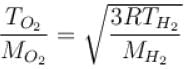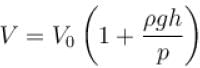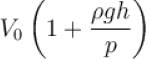Kinetic Theory Of Gases MCQ Level – 2 (part - 2) - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Kinetic Theory Of Gases MCQ Level – 2 (part - 2)
An ideal gas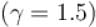 is expanded adiabatically. How many times the gas to be expanded to reduce the rms velocity of molecules 2 times.
is expanded adiabatically. How many times the gas to be expanded to reduce the rms velocity of molecules 2 times.
Select one:
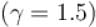 is expanded adiabatically. How many times the gas to be expanded to reduce the rms velocity of molecules 2 times.
is expanded adiabatically. How many times the gas to be expanded to reduce the rms velocity of molecules 2 times.Select one:
A box containing N molecules of a perfect gas at temperature T1 and pressure p1.
The number of molecules in the box is doubled keeping the total kinetic energy of the gas same as before. If the new pressure is p2 and temperature T2, then.
Select one:
The number of molecules in the box is doubled keeping the total kinetic energy of the gas same as before. If the new pressure is p2 and temperature T2, then.
Select one:
Three closed vessels A, B and C are at the same temperature T and contain gases which obey Maxwellian distribution of velocities. Vessel A contains only O2 , B only N2 and C a mixture of equal quantities of O2 and N2. If the average speed of O2 molecules is vessel A is v1 , that of the N2 molecules in vessel R is v2 , the average speed of O2 molecule in vessel C is (where M is the mass of an oxygen molecule)
Select one:
Select one:
Gas at pressure p0 contained in a vessel. If the masses of all the molecules are halved and their speeds are doubled, the resulting pressure p will be equal to.
Select one:
Cooking gas containers are kept in a lorry moving with uniform speed. The temperature of the gas molecule inside will.
Select one:
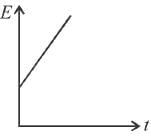
The graph which represents the variation of mean kinetic energy of molecules with temperature t°C is.
Select one:
At what temperature is the root mean square velocity of gaseous hydrogen molecules is equal to that of the oxygen molecules at 47°C.
Select one:
An air bubble of volume V0 is released by a fish at a depth h in a lake. The bubble rises to the surface. Assume constant temperature and standard atmosphere pressure P above the lake. The volume of the bubble just before touching the surface will be (density of water is ρ.
Select one:
A gas is filled in the cylinder shown below. The pistons are joined by a string. If the gas is heated, the piston will.
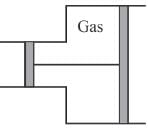
Select one:
The figure below shows graph of pressure and volume of a gas at two temperatures T1 and T2 which of the following inferences is correct?
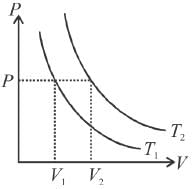
Select one:


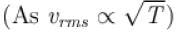
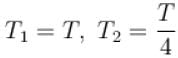
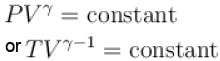
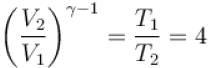
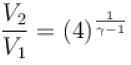
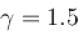
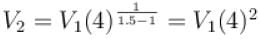
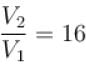
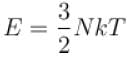
 and finally
and finally 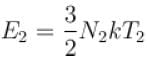
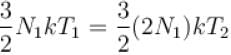
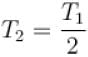
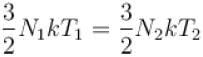
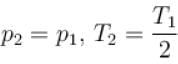

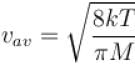
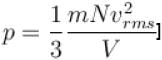
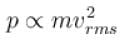
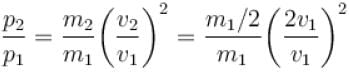
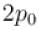
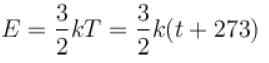
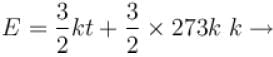 Boltzmann constant
Boltzmann constant