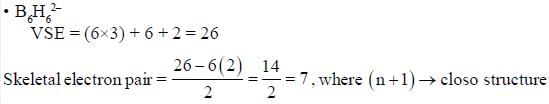Minor Test - Inorganic Chemistry - GATE Chemistry MCQ
30 Questions MCQ Test GATE Chemistry Mock Test Series - Minor Test - Inorganic Chemistry
The intense blue color of Type - 1 Copper protein is due to
The number of Zn atoms p resent in alcohol dehydrogenase enzyme is are_____________
The pair of lanthanoids showing strong emission spectrum are
Select Correct Statement from following regarding t-block elements
(i) A bsorption spectrum of lanthanoids(III) is largely dependent on nature of ligands.
(ii) Magnetic moments of lanthanoids (III) is generally greater than corresponding actinides (III)
(iii) Spectra of Heavier actinides (III) ions show resemblance with lanthanoids (III) ions
(iv) Ce3+ ion is colourless in aqueous medium.
The correct answer is
Which among the following alkene will bind most strongly to a metal?
The product which formed on reaction of Cu with dilute HNO3 is
H4P2O6, H4P2O7, H2S2O8, H2SO5
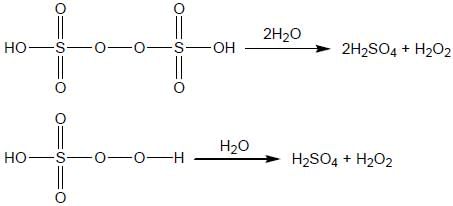
The total number of species that will produce H2 O2 on hydrolysis are _____________
Among the following boron hydrides which of the following is highest bronsted acidity :
The reaction of [Co(CN)5Cl]3- with -OH follows______ order reaction with respect to -OH.
Which of the following oxides have lowest Neel temperature ?
Value of β (nephelauxetic parameter) is maximum for:
In the trigonal prismatic crystal field, the d-orbital with the highest energy is
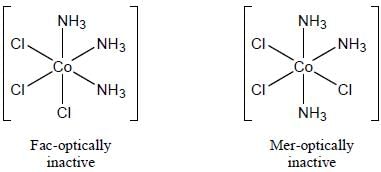
The number of isomers possible for [Co(NH3)3 Cl3] is are____________
In the transformation of 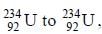 emission is an a-particle. what would be the other emission.
emission is an a-particle. what would be the other emission.
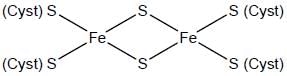
The number of inorganic sulfide(s) in photosynthetic terredoxin is are___________.
The correct order of second ionization potential among the following elements : Be, B, C, N and O is
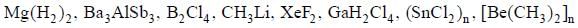
The number of species shows 3c - 4e- bonding is are______________.
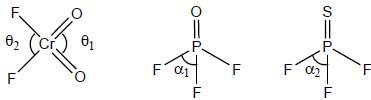
Identify the correct relation for the bond angle:
The projection of the orbital angular momentum and reflection through a plane containing intemuclear axis for first excited state of O2 are:
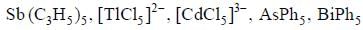
The number of species having dx2 - y2 orbital involved in hybridisation is are _________________
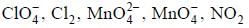
The number of species that im dergoes dispropoitionation in an alkaline medium is______________
The collect order of acidity of the trioxides is:
The correct order of reactivity of GaCl3, A1C13 and BC13 with soft Lewis bases is :
The collect order of thermal stability of the following boron compound is
|
20 docs|37 tests
|



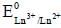 is
is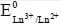 value also increases.
value also increases.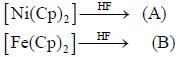
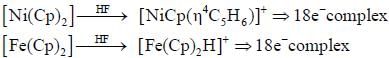
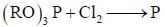
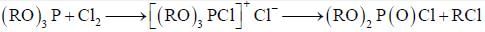
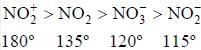
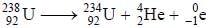

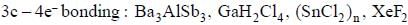
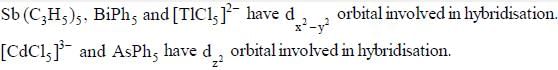
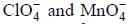 are in their maximum oxidation state, doesn't undergo dispropoitionation reaction while
are in their maximum oxidation state, doesn't undergo dispropoitionation reaction while  species undergo dispropoitionation in alkaline medium.
species undergo dispropoitionation in alkaline medium.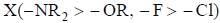 to form a π-bond with B.
to form a π-bond with B.