Multiple Choice Questions (MCQs): Chemical Kinetics - NEET MCQ
25 Questions MCQ Test - Multiple Choice Questions (MCQs): Chemical Kinetics
Which of the following statement is true for order of a reaction?
For the first order reaction, half life is equal to
Straight line graph for first order reaction is obtained between
The effect of temperature on reaction rate is given by
The chemical reaction in which reactants require high amount of activation energy are generally
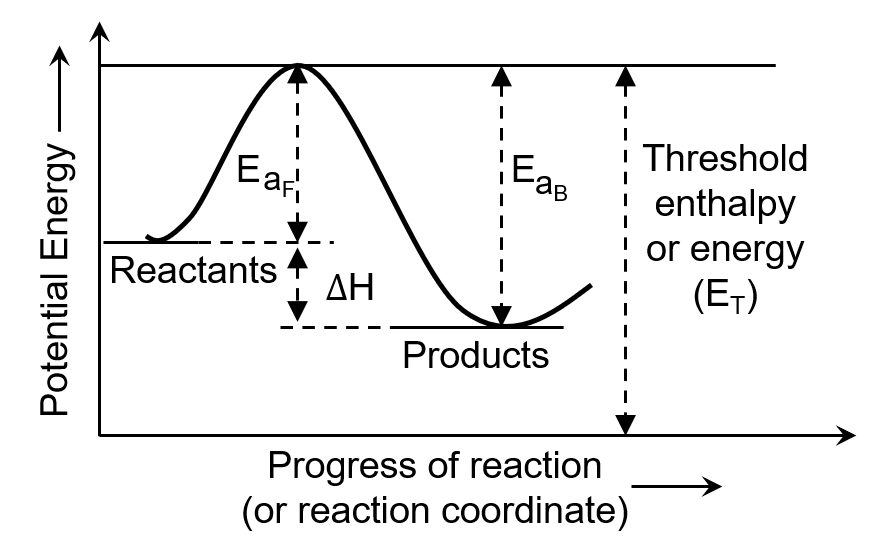
The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is called
The expression which relates the rate of reaction to the concentration of the reactants are called?
The constant k used in rate equation is known as
The radioactive isotope used in determining the age of organic substances is
The substance that slows down the reaction without being consumed is known as
Which among the following statement is not true for catalyst?
A first order reaction is 50% completed in 1.26 × 1014 s. How much time would it take for 100% completion?
Collision theory is applicable to
Rate of reaction does not remain constant throughout because
The unit of rate constant for a first order reaction is
Reaction which takes place in one step is known as
Which among the following statement is not true for rate constant of a reaction?
Which among the following is an example of pseudo first order reaction?
Which among the following is an example of first order reaction?
The rate law for the reaction 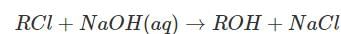 is given by rate = k[RCl]. The rate for this reaction
is given by rate = k[RCl]. The rate for this reaction
For an endothermic reaction, the minimum value for the energy of activation in terms of will be
Order of the photochemical reaction occurring between hydrogen and chlorine is
The temperature coefficient of most of the reactions lies between
Following mechanism has been proposed for a reaction
The rate law expression for the reaction is
The expression which gives 3/4th life of first order reaction is