NVS PGT Chemistry Mock Test - 1 - NVS TGT/PGT MCQ
30 Questions MCQ Test - NVS PGT Chemistry Mock Test - 1
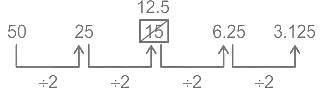
Find the wrong term in the given series?
50, 25, 15, 6.25, 3.125
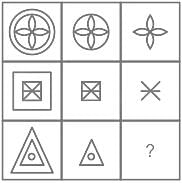
Study the figures in given matrix and find out the answer figure which completes the problem figure matrix.
In the number 76534218, each digit is replaced by the next digit i.e., '1' is replaced by '2', '2' is replaced by '3' and so on and then the digits are arranged in ascending order from left to right, which digit will be fifth from the left end?
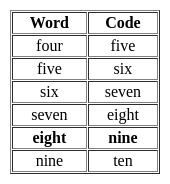
If in a certain code language, 'four' is called 'five', 'five' is called 'six', 'six' is called 'seven', 'seven' is called 'eight', 'eight' is called 'nine' and 'nine' is called 'ten'. Then what is cube of the number 2?
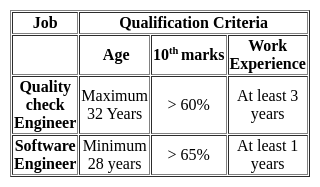
To qualify for a post of Quality check Engineer in an IT company, the maximum age can be of 32 years, 10th marks should be more than 60% and work experience should be at least 3 years. To qualify for Software Engineer, one should have a minimum age of 28 years, 10th marks should be more than 65%, and work experience of at least 1 year. Rajat has 64% marks in 10th an age of 27 years and work experience of 5 years. For which of these jobs does he qualify?
One of the main technological edges of ICT involves in enhancing interactive communication among people staying at varied locations through electronic devices. This technology is called as?
Shalini investigates a topic thoroughly and does not need to be over-directed. In which learning phase is she?
Only One Option Correct Type
This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
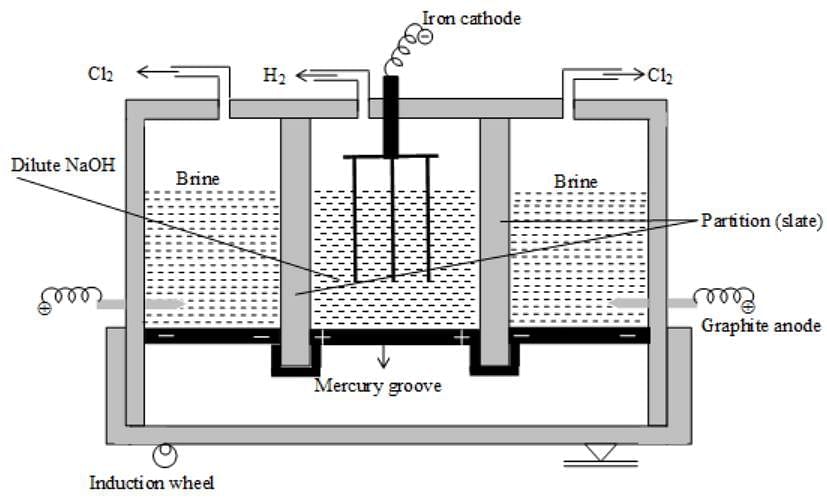
During the electrolysis of aqueous Zn(NO3)2 solution
Direction (Q. Nos. 1 - 6) This section contains 6 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
A pure enantiomer with molecular formula C6H13OBr, when reacted with PBr3, an achiral product C6H12Br2 is obtained that has no chiral carbon. The compound which satisfy this condition could be (no bond to chiral carbon is broken during the reaction)
ΔHvap = 30 kJ mol-1 and ΔSvap = 75 J mol-1 K-1. Thus, temperature of vapour at one atmosphere is
[IIT JEE 2004]
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. The equilibrium which is not affected by volume change at constant temperature is
The atom which defines the structure of a family of organic compounds and their properties is called ___________
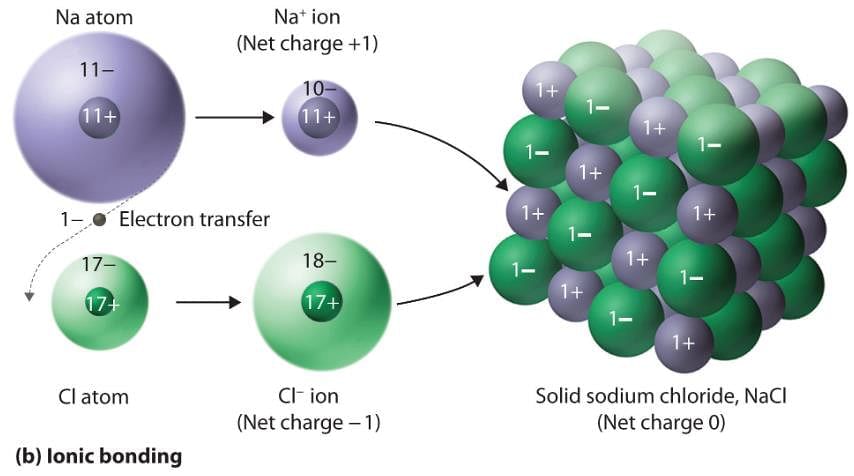
In which of the following solids, ions of opposite charges are held together by strong electrostatic forces of attraction?
Which of the following are not functional isomers of each other?
Which among the following has square pyramidal geometry?
Osmotic pressure of 40% (wt./vol.) urea solution is 1.64 atm and that of 3.42% (wt./vol.) cane sugar is 2.46 atm. When equal volumes of the above two solutions are mixed, the osmotic pressure of the resulting solution is -
Improve the bracketed part of the sentence with the parts given below.
Q. He (turned over a new leaf) after serving his remaining prison sentence





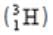 has 1 proton and 2 neutrons.
has 1 proton and 2 neutrons.