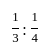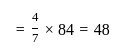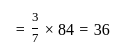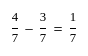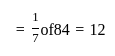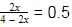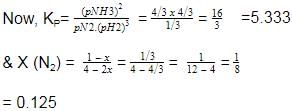NVS PGT Chemistry Mock Test - 6 - NVS TGT/PGT MCQ
30 Questions MCQ Test - NVS PGT Chemistry Mock Test - 6
A profit of Rs. 84 is divided between A and B in the ratio of  . What will be the difference in their profits?
. What will be the difference in their profits?
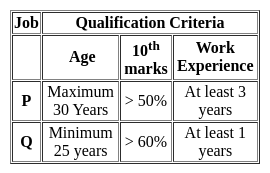
To qualify for a job P, maximum age can be of 30 years, 10th marks should be more than 50% and work experience should be at least 3 years. To qualify for job Q, one should have a minimum age of 25 years, 10th marks should be more than 60% and work experience of at least 1 year. Rahul has 48% marks in 10th an age of 28 years and. work experience of 5 years. For which of these jobs does he qualify?
Arrange the following words in the logical and meaningful order.
1. Designing
2. Manufacturing
3. Production
4. Planning
5. Implementation
A statement is given followed by two conclusions. Find which conclusion(s) is /are true based on the given statement.
Statements:
K > M < L = Z = O < P
Conclusions:
I. P < Z
II. M = Z
Jatin, born on 05 January 1998, is in final year of Engineering of an Integrated course, with an aggregate of 75% till his 7th semester, he does not have any permanent body tattoos.
Which command moves the turtle to another position or the screen without drawing a line?
Which of the following would be the characteristic of an effective teacher?
How many atoms of hydrogen are in 67.2 L of H2 at STP?
Consider the ground state of Cr atom (Z = 24). The numbers of electrons with the azimuthal quantum numbers, = 1 and 2 are, respectively:
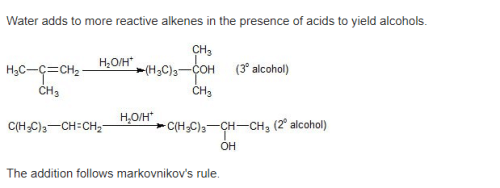
Acid catalyzed hydration of alkenes except ethene leads to the formation of –
[AIEEE-2005]
At 700 K and 350 bar, a 1 : 3 mixture of N2(g) and H2(g) reacts to form an equilibrium mixture containing X (NH3)= 0.50. Assuming ideal behaviour Kp for the equilibrium reaction,
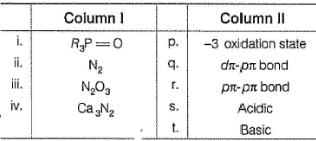
Match the Column I with Column II and mark the correct option from the codes given below :
When K2O is added to water, the solution becomes basic in nature because it contains a significant concentration of -
Sodium pentacyanonitrosylferrate(II) is also called?
For the process, and 1 atmosphere pressure, the correct choice is
[JEE Advanced 2014]
What are the basic constituent particles forming diamond crystals?
Which of the following forms a colloidal solution in water?
The complex [Co(NH3)6[Cr(C2O4)3] and [Cr(NH3)6[Co(C2O4)3] exhibit
Direction:
This section contains 5 questions. When worked out will result in an integer from 0 to 9 {both inclusive).
Q.
Relative decrease in vapour pressure of an aqueous NaCI solution is 0.167. Thus, number of moles of NaCI present in 180 g of H20 is ... .
Action research is different from basic research because
In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.
Q. A story that can be interpreted to reveal a hidden meaning.
In the following question, a sentence has been given in Active/Passive Voice. Out of the four alternatives suggested, select the one which best expresses the same sentence in Passive/Active Voice.
I have seen him.