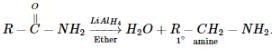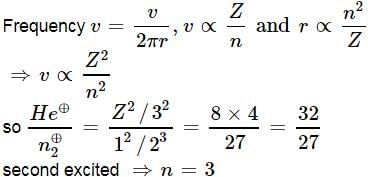NVS PGT Chemistry Mock Test - 7 - NVS TGT/PGT MCQ
30 Questions MCQ Test - NVS PGT Chemistry Mock Test - 7
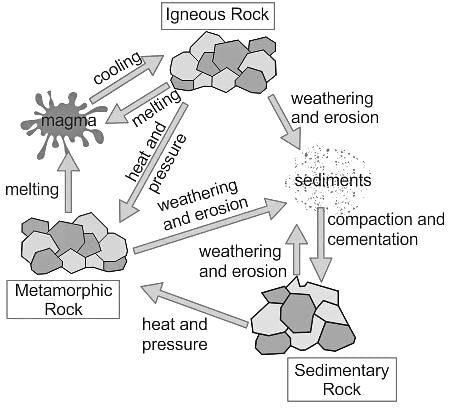
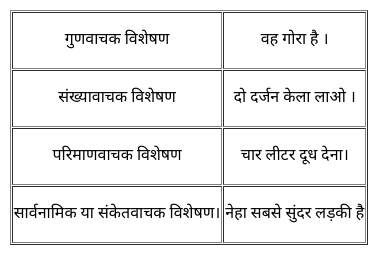
Soil contains decayed remains of living organisms. This is called ______.
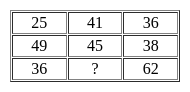
Select the number which can be placed at the sign of the question mark (?) from the given alternatives.
The positions of how many digits in the number 837912 will remain unchanged after the digits within the number are rearranged in descending order (from left to right)?
Which of the following applications is used to create presentations?
What is the most effective way to discipline a mischievous student?
Effective teaching is by and large a function of teacher's
Which among the following is a non-reducing sugar?
The Aufbau principle states : In the ground state of the atoms, the orbitals are filled in order of
Aniline does not undergo Friedel – Crafts reaction
Statement I : cis-2-butene with cold, dilute, alkaline KMnO4 gives meso-2,3- butanediol.
Statement II : In alkaline solution, under cold condition, KMnO4 acts as a mild oxidising agent.
Which of the following statement is true about Langmuir’s adsorption?
A metal crystallizes with a face-centered cubic lattice. The edge of the unit cell is 408 pm. The diameter of the metal atom is
The physical properties of isotopes differ due to:
PCl5 dissociation a closed container as :
PCl5(g) 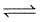 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is α, the partial pressure of PCl3 will be :
Which reaction can be used for the direct conversion of amides into 10 amine ?
At 675 K, H2(g) and CO2(g) react to form CO(g) and H2O(g), Kp for the reaction is 0.16. If a mixture of 0.25 mole of H2(g) and 0.25 mol of CO2 is heated at 675 K, mole% of CO(g) in equilibrium mixture is :
Which of the following acids does not exhibit optical isomerism?
Ratio of frequency of revolution of electron in the second excited state of He⊕and second state of hydrogen is
Which of the following student reveals the "Logical Mathematical" intelligence, as proposed by Gardner?
The following sentence has been broken into four parts with an error in one part. Identify that part and mark it as your answer. If there are no errors in any of the given parts, mark option 4 or ‘No error’ as your answer.
Q. The metro rail management apologized (1)/ for the inconsistency it had caused (2)/ to all the commuters. (3)/ No error (4).
The following sentence has been broken into four parts with an error in one part. Identify that part and mark it as your answer. If there are no errors in any of the given parts, mark option 4 or ‘No error’ as your answer.
Q. I will not be (1)/ able to make it (2)/ to the function tomorrow.(3)/ No error(4).