NVS PGT Chemistry Mock Test - 8 - NVS TGT/PGT MCQ
30 Questions MCQ Test - NVS PGT Chemistry Mock Test - 8
Which of the following is not a department under Ministry of Finance?
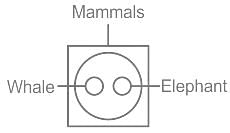
Identify the diagram that best represents the relationship among the given classes. Mammals, Whale, Elephant
Choose the alternative which is an odd word/number/letter pair out of the given alternatives.
What are the steps taken to open an existing document?
Internet access by transmitting digital data over the wires of a local telephone network is provided by
How do you create a blank line between two lines in a document?
Solid X is a very hard electrical insulator in solid as well as in molten state. It melts at extremely high temperature. Solid X is a
In which of the following processes, does the value of magnetic moment change ?
In the following alcohol, which — OH group is involved to the maximum extent in H-bonding?
The element which shows only negative oxidation state/s among following elements is:
The hydrocarbon which can react with sodium in liquid ammonia is -
[AIEEE 2008]
Lyophilic colloids that shows the property of protecting lyophobic colloids are also called as:
25.3 g of sodium carbonate, Na2CO3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, molar concentration of sodium ions, Na+ and carbonate ions, 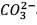 are respectively. (At. mass of carbon = 40)
are respectively. (At. mass of carbon = 40)
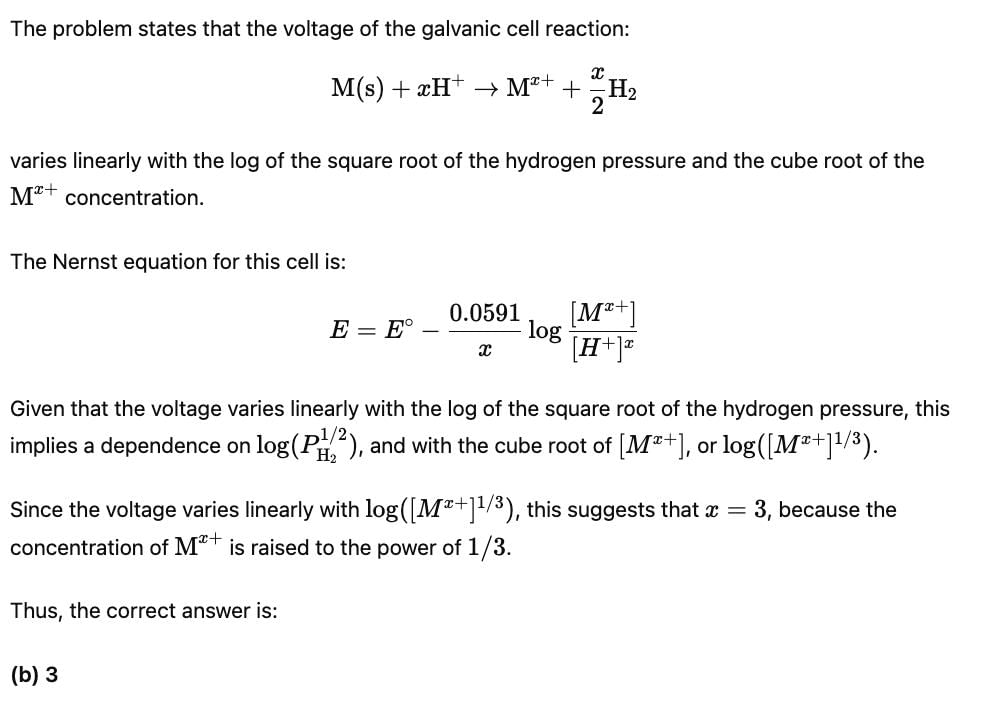
It is observed that the voltage of a galvanic cell using the reaction M(s) + xH+→ Mx+ + X/2H2 varies linearly with the log of the square root of the hydrogen pressure and the cube root of the Mx+ concentration. The value of x is
The standard enthalpies of formation of SF6 (g), S (g)and F (g) are -1100, + 275 and + 80 kJ mol-1. Thus, average bond energy of (S—F) in SF6 is
The alkali metals are low melting. Which of the following alkali metal is expected to melt if the room temperature rises to 30°C?
In an atom, an electron is moving with a speed of 600 m/s with an accuracy of 0.005%. Certainity with which the position of the electron can be located is (h = 6.6×10-34 kg m2s-1 ,mass of electron, em = 9.1×10-31 kg ) [AIEEE 2009]
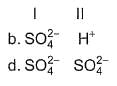
Consider the following reactions,
I. Zn + dil. H2SO4 → ZnSO4 + H2
II. Zn + conc. H2SO4 → ZnSO4+ SO2 + H2O
Oxidising agents in I and II are
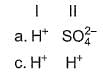
Lead emitted by vehicles interferes with development of:
Which of the following strategies will work for mentally retarded children?
Which of the following is proposed in National Education Policy 2020?
(i) Learning how to learn
(ii) Increasing course content
(iii) 360-degree holistic progress report card
(iv) Standardized curriculum, pedagogy, and assessment
Choose the correct option.



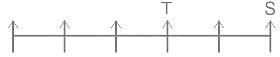
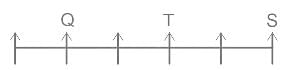
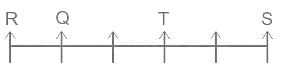
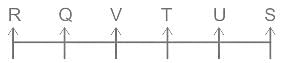
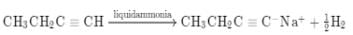
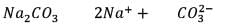
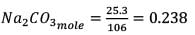
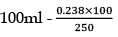
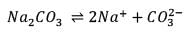
 0.955
0.955