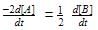NVS PGT Chemistry Mock Test - 9 - NVS TGT/PGT MCQ
30 Questions MCQ Test - NVS PGT Chemistry Mock Test - 9
Which among Akbar's Navratna was involved in creating a land revenue system?
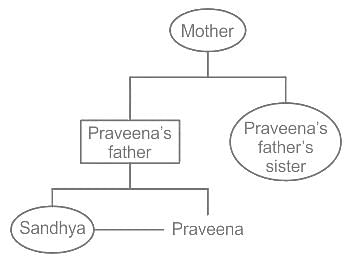
Sandhya is sister of Praveena. How is Praveena’s father’s sister’s mother related to Sandhya?
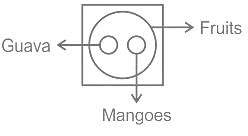
Select the Venn diagram that represents the given set of classes.
Guava, Mangoes, Fruits
Which of the following statements hold(s) true about MPEG standards?
Statement 1: It is a set of standards for audio and video compressions.
Statement 2: It was developed by Motion Pixel Expert Group.
For a reaction 1/2 A→ 2B, rate of disappearance of ‘A’ related to the rate of appearance of ‘B’ by the expression -
[AIEEE 2008]
In which of the following molecules would you expect the N to N bond to be shortest ?
When Al2O3 is electrolysed ,cation and anions are discharged. For a given quantity of electricity,ratio of number of moles of Al and O2 gas is
Which of the ligand can show linkage isomerism and acts as flexidentate ligand:
For the following equilibrium starting with 2 moles SO2 and 1 mole O2 in 1 L flask,
Equilibrium mixture required 0.4 mole in acidic medium. Hence, Kc is
In which of the following the stability of two oxidation states is correctly represented?
Which representation for the Lewis structure of HNO3 is correct?
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct
Q.
Match the different solutions in Column I with their ΔTb in Column II and select the answer from the codes given below.
Direction (Q. Nos. 20-21) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q.
Consider the molecules in Column I and match them with their stereochemical properties from Column II

What compound is produced when cyclohexene is treated with concentrated KMnO4?
What is the order of a reaction which has a rate expression ; Rate =
The conversion of primary aromatic amines into diazonium salts is known as:
Kritika who does not talk much at home talks a lot at school. It shows that:
In the following question, four words are given out of which one word is incorrectly spelled. Find the incorrectly spelled word.
In the following question, a sentence has been given in Active/Passive Voice. Out of the four alternatives suggested, select the one which best expresses the same sentence in Passive/Active Voice.
Q. Does he want this?
In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.
A practical person
निम्नलिखित वाक्य में प्रधान उपवाक्य है:
मेरा यह सोचना कि लोकेश प्रतिभाशाली छात्र है, सही निकला।



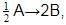 rate of disappearance of A is related to rate of appearance of B by the expression:
rate of disappearance of A is related to rate of appearance of B by the expression: