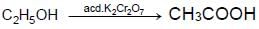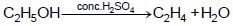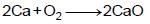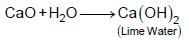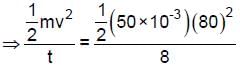National Level Solved Paper (SAT)- 2020-21 - Class 10 MCQ
30 Questions MCQ Test - National Level Solved Paper (SAT)- 2020-21
The plasma membrane (pm) forms the boundary of lung cells.
Which of the following statements is true for the pm?
A. pm is a semipermeable membrane
B. Water moves across the pm by Osmosis.
C. O2 and CO2 can cross the pm by diffusion.
D. Na+ and K+ ions can pass the pm by diffusion.
Which of the following statements is true for the pm?
A. pm is a semipermeable membrane
B. Water moves across the pm by Osmosis.
C. O2 and CO2 can cross the pm by diffusion.
D. Na+ and K+ ions can pass the pm by diffusion.
Eukaryotic cells contain several membrane bound subcellular structures called Organelles. The vacuole is one such organelle found in both animal and plant cells.
Which of the following statement are true for vacuoles?
A. Contain cell sap.
B. Provide turgidity to the plant cell.
C. Plant cell vacuoles are smaller than animals cell vacuoles.
D. Vacuoles store amino acids, sugar, acids and contain protein.
Which of the following statement are true for vacuoles?
A. Contain cell sap.
B. Provide turgidity to the plant cell.
C. Plant cell vacuoles are smaller than animals cell vacuoles.
D. Vacuoles store amino acids, sugar, acids and contain protein.
What is the reason for the Cardiac muscles not getting fatigued?
Grafting is possible among dicot plants but not in monocot plants. This is due to presence of one of the following conditions in dicot plant.
Parenchyma, collenchymas and sclerenchyma are kinds of simple permanent tissues in plants. Which of the following statement is true for collenchymas?
A. Made up of dead cells.
B. Have very little interecellular space
C. Cells are irregularly thickened at the corners
D. Cell wall contains lignin
Trees of the genus Pinus are placed in higher groups compared to those of Marsilea genus because of the presence of one of the following features.
A bulb of (220 V, 60 W) is operated on 110 V supply then power developed in it is
What will happen to cells of cyanobacteria if they are placed in purified water?
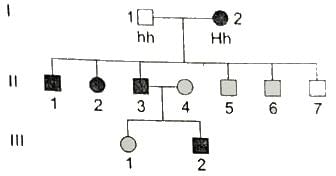
Hutington’ s disease is an autosomal disorder characterized by movement, cognitive and psychiatric disorders. Study the given pedigree and identify the genotype of II - 3 and II - 4.
[Note : Solid squares/circles represent affected individuals and empty squares / circles denote unaffected normal individuals.]
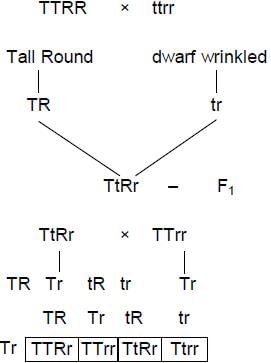
When a tall plant with round seeds was hybridized with a dwarf plant with wrinkled seeds; all offspring in F1 generation were tall plants that produced round seeds. As per Mendel’ s law of independent assortement, what percent of offspring will produce wrinkled seeds if F1 is crossed with tall plant producing wrinkled seeds?
What would happen to earth if carbondioxide was absent from its atmosphere?
The following figure represents the flow of energy in a pyramid of food. If this ecosystem receives 100000 kcal of sunlight energy of the energy finally available to Tertiary Consumer (TC) is:
Pollen grains of a fruiting plants species are deposited on the female flower by a pollinator. However, the female flower does not get fertilized. Which of the following observation is true?
The values of stoichiometirc coefficients m, x, y and z in the following reaction after balancing are, respectively:
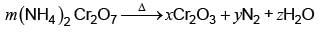
Identify the incorrect statement for the reaction 2H2S + SO2 → 3S + 2H2O is : (Atomic mass of S = 32)
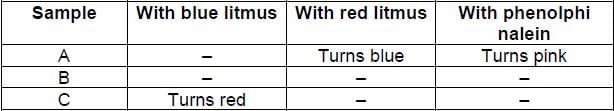
You are provided with aqueous solutions of three salts A, B and C. 2 -3 drops of blue litmus solution, red litmus solution and phenolphinalein were added to each of these solutions in separate experiments. The change in colour of different indicators were recorded in the following table:
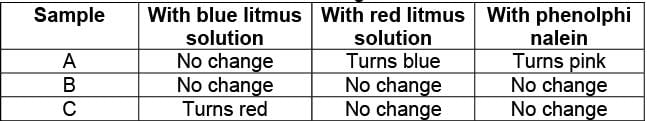
On the basis of above observations, identify A, B and C from the following options:
The plasma membrane (pm) forms the boundary of lung cells.
Which of the following statements is true for the pm?
A. pm is a semipermeable membrane
B. Water moves across the pm by Osmosis.
C. O2 and CO2 can cross the pm by diffusion.
D. Na+ and K+ ions can pass the pm by diffusion.
The compound ‘ A’ when treated with alkaline potassium permanganate gives ‘ B’ , and with conc. sulphuric acid gives ‘ C’ and ‘ D’ . The compunds A, B, C and D are respectively.
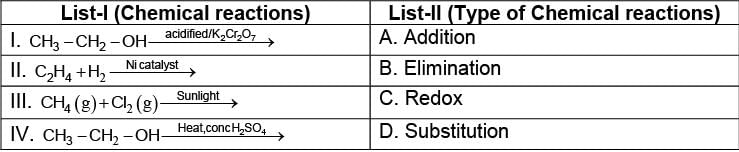
Match the chemical reaction given in the List-I with type of chemical reactions given in the List-II and select the correct answer from the options given below:
Two beakers A and B contain iron (II) sulphate solution. In the beakers A and B, small pieces of copper and zinc are placed respectively. It is found that a grey deposit forms on the zinc but not on the copper. From these observations, it can be concluded that:
Sulphur powder is heated on a spatula. A piece of both, moist blue and red litmus papers are brought one by one near the gas evolved during heating. The action of gas on the moist litmus papers will be :
Two samples A and B of a pure substance containing elements Y and Z are obtained from two different sources. 5g of sample A contains 1.25 g of Z. Sample B is made of 75% of Y by weight. This is an illustration of which of the following laws?
An element X with atomic number 13 combines with another element Y of atomic number 17. The formula of the compound formed and nature of bond will be:
Select the correct options from the following statements:
(i)  are isobars of each other.
are isobars of each other.
(ii)  to form a product which contains ionic bonds.
to form a product which contains ionic bonds.
(iii)  are isobars of each other.
are isobars of each other.
(iv)  to form a compound whose aqueous solution is known as lime water.
to form a compound whose aqueous solution is known as lime water.
Identify the correct order of atomic radii of following elements:
Which of the following statements are true?
I. On heating the kinetic energy of particles in solids does not change because they have a fixed position.
II. Sublimation is the change of gaseous state directly to solid state without going through liquid state and vice versa.
III. The movement of particles from an area of higher concentration to lower concentration is called diffusion.
IV. The rate of evaporation is not affected by increasing the temperature.
A train moving at uniform 90 km/h is approaching a flag station whose platform is 500 m long. Station master is standing at the centre of the platform. Train starts blowing whistle when engine is 1 km away from near end of the platform and continues blowing whistle till engine crosses of the platform without stopping. If the speed of the sound is assumed to be 300 m/s, then the duration for which station master hears the whistle is?
A swimmer can swim in still water at a speed of 15 km/h. A river is flowing at 5 km/h. The swimmer starts from a point and swim 1 km upstream and then returns by swimming downstream back to original position. During this, the average speed of his/her swimming is :
A car P is moving with a uniform speed of 72 km/h towards another car Q at rest on a straight level road. At a particular instant when the distance between P and Q is 525 m the car Q started accelerating at 2 m/s2 towards P. Find the distance traveled by Q, when both the cars meet.
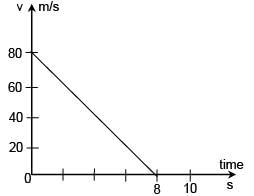
Figure shows the velocity versus time graph for a block of mass 50 g sliding on a rough floor. The average rate at which energy dissipates (in J/s) due to the force of friction is :



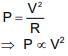

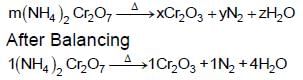
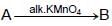 (I)
(I)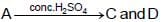 (II)
(II)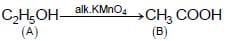 {Oxidation of alcohol}
{Oxidation of alcohol}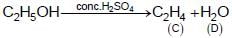 {Dehydration of alcohol}
{Dehydration of alcohol}