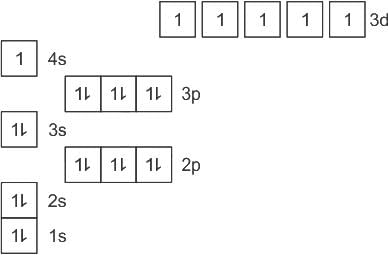
The answer is option 4
Key Points Hückel's Rule of aromaticity is a simple way to determine if a planar ring molecule might display aromatic properties. According to this rule, a ring molecule is aromatic if it is:
- Cyclic: The molecule must consist of a ring of atoms.
- Planar: The atoms in the molecule must all lie in the same plane.
- Conjungated: The molecule must have a continuous ring of overlapping p orbitals, allowing for delocalization of π electrons.
- Following the 4n+2 rule: The molecule must have a particular number of π electrons. Specifically, the total count of π electrons (those found in the overlapping p-orbitals) should be such that it fits the expression 4n+2, where n is any non-negative integer (including zero). If the quantity of π electrons can fit this formula, the molecule is aromatic.
Additional Information
Hexene is an alkene, not an aromatic compound. Alkenes are hydrocarbons that contain at least one carbon-carbon double bond, but they do not have the special properties of aromaticity.
Aromatic compounds, in contrast, are organic compounds that contain a ring of continuously overlapping p-orbitals, like benzene. Aromatic molecules are unusually stable, and this stability, termed aromaticity, can be predicted based on the Hückel rule.
Hückel's rule states that a compound is aromatic if it has a continuous ring of p orbitals, is planar, is cyclic, and follows the 4n+2 rule, where n is a non-negative integer. The 4n+2 rule refers to the number of pi electrons in the compound.
- Benzene (C6H6) has six pi electrons, satisfying the 4n+2 rule where n=1. It is a classic example of an aromatic compound.

it follows 4n+2 rule, where n is 1- Naphthalene (C10H8) has 10 pi electrons, satisfying the 4n+2 rule where n=2. Therefore, it is also an aromatic compound.
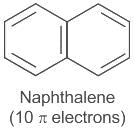
it follows 4n+2 rule, where n is 2- Anthracene (C14H10) has 14 pi electrons, satisfying the 4n+2 rule where n=3. It is also a distinctively aromatic compound.
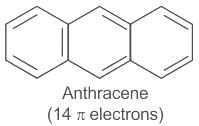
it follows 4n+2 rule, where n is 3- hexene is non aromatic

it not planer
In contrast, Hexene does not have a ring of overlapping p-orbitals and does not follow the 4n+2 rule (since it does not have a ring structure at all)—showing that it does not possess aromaticity per Hückel’s rule.
Conclusion:-
So, Hexene is not an aromatic compound.





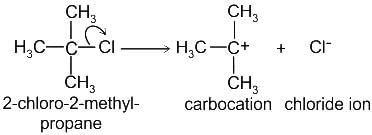
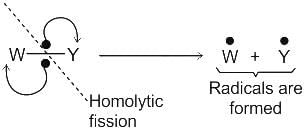




 .
.