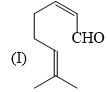
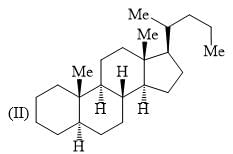
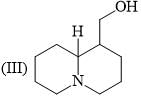
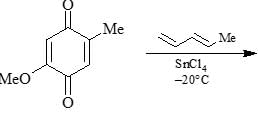
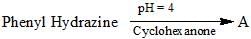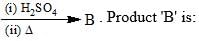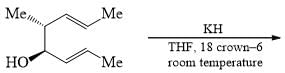Organic Analysis - Chemistry MCQ
30 Questions MCQ Test - Organic Analysis
Which of the following compound is expected to show a sharp singlet for one of its protons at δ ≥ 8 ppm in 1H NMR spectrum, given that this signal remains unaffected on shaking the solution thoroughly with D2O?
Sometimes, the color observed in Lassaigne’s test for nitrogen is green. It is because of:
Arrange the following molecules, in their chemical shift value(s) of ortho hydrogen in 1H NMR:
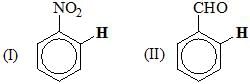
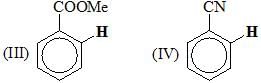

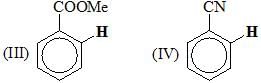
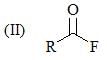
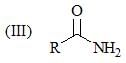
Consider the following:
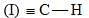
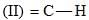
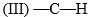
Order of C-H stretching frequency in IR-spectroscopy would be:
The complementary strand of DNA for the following single stranded DNA sequence: 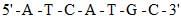 is:
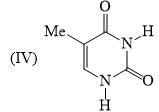
is:
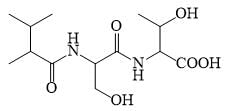
The correct sequence of the amino acids present in the tripeptide given below is:
Consider the following:
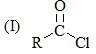
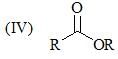
Order of stretching frequency in IR-spectroscopy would be:
For the detection of sulfur in an organic compound, Lassaigne’s extract is acidified with acetic acid. Lead acetate solution is then added to it. The black precipitate formed is due to the formation of ?
Which of the following compound show only two signals in 1H NMR and a strong IR bond at
~1690 cm–1:
A compound with molecular formula C5H10O does not form, 2, 4-DNP derivative. It responds positively to Iodoform test and on ozonolysis gives one mole formaldehyde. It could be:
The correct match of the 1H NMR chemical shift d of the following species/compound is:
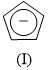

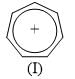
In the Lassaigne’s test for the detection of nitrogen in an organic compound, the Prussian blue colour is due to the formation of _______________:
The correct order of 1H NMR chemical shift (δ) values for the labeled methyl groups in the following compound is:
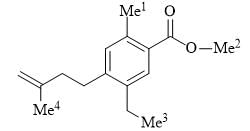
The Beckmann rearrangement of a bromo-acetophenone oxime (C8H8BrNO) gives a major product having the following 1H NMR – 9.85 (S, 1H), 7.88 (S, 1H), 7.45 (d, 1H, J=7.2), 7.17 (m, 1H, 7.12 (d, 1H, J=7.0 Hz), 2.06 (S, 3H). The structure of the product is:
The absorption at lmax 279 nm (ε = 15) in the UV spectrum of acetone is due to:
The structure of D-(+)-glucose is:
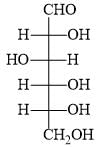
The structure of L-(–)-glucose is:
Which of the following compounds will behave as a reducing sugar in an aqueous KOH solution?
Consider the following:
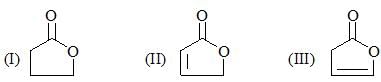
Order of their stretching frequency in IR-spectroscopy would be:
In the UV-visible absorption spectrum of an α, β-unsaturated carbonyl compound, with increasing solvent polarity:
Among the following, the compound that displays an IR band at 2150 cm–1 is:
The compound which shows IR frequencies at both 3314 and 2126 cm–1 is:
Compound I gives a strong infrared absorption at 1730 cm–1. 1H NMR spectrum indicates that it has two types of hydrogen atoms; one H atom appearing as singlet at δ = 9.7 ppm and 9H atoms appearing as a singlet at δ = 1.2 ppm. The structure of I is:
Consider the following:

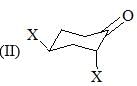
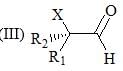
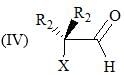
Order of C-O stretching frequency in IR-spectroscopy would be:
During the detection of sulfur in an organic compound, the violet color formed by the addition of Sodium nitroprusside to Lassaigne’s extract is due to _____________: